J Periodontal Implant Sci.
2012 Jun;42(3):95-104. 10.5051/jpis.2012.42.3.95.
Heat or radiofrequency plasma glow discharge treatment of a titanium alloy stimulates osteoblast gene expression in the MC3T3 osteoprogenitor cell line
- Affiliations
-
- 1Hospital for Special Surgery affiliated with the Weill Medical College of Cornell University, New York, NY, USA. dem14@columbia.edu
- 2General Medical Research, James J. Peters VA Medical Center, Bronx, NY, USA.
- 3Langmuir Center for Colloids and Interfaces, Columbia University, New York, NY, USA.
- KMID: 2094733
- DOI: http://doi.org/10.5051/jpis.2012.42.3.95
Abstract
- PURPOSE
The purpose of this study was to determine whether increasing the Ti6Al4V surface oxide negative charge through heat (600degrees C) or radiofrequency plasma glow discharge (RFGD) pretreatment, with or without a subsequent coating with fibronectin, stimulated osteoblast gene marker expression in the MC3T3 osteoprogenitor cell line.
METHODS
Quantitative real-time polymerase chain reaction was used to measure changes over time in the mRNA levels for osteoblast gene markers, including alkaline phosphatase, bone sialoprotein, collagen type I (alpha1), osteocalcin, osteopontin and parathyroid hormone-related peptide (PTH-rP), and the osteoblast precursor genes Runx2 and osterix.
RESULTS
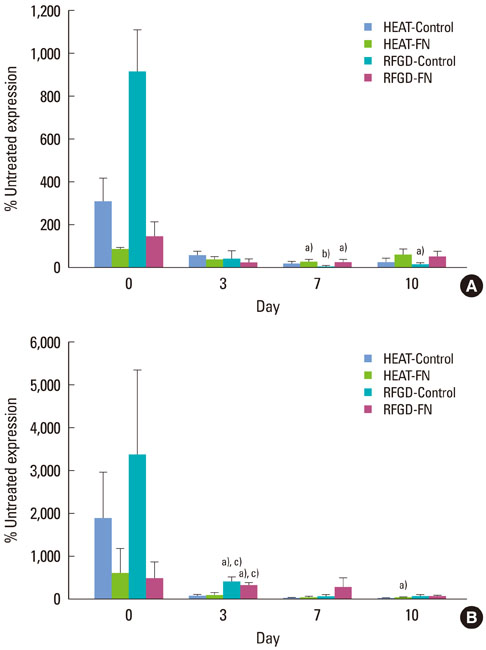
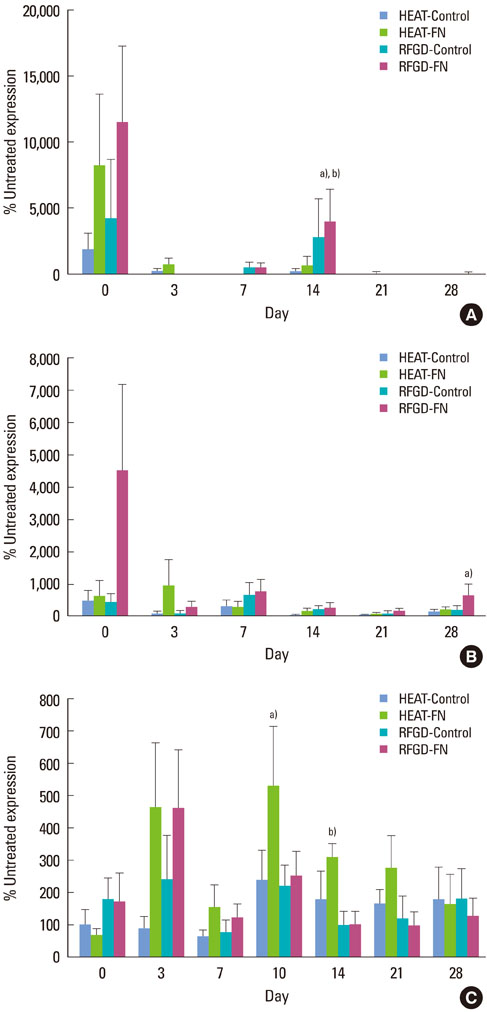
Osteoprogenitors began to differentiate earlier on disks that were pretreated with heat or RFGD. The pretreatments increased gene marker expression in the absence of a fibronectin coating. However, pretreatments increased osteoblast gene expression for fibronectin-coated disks more than uncoated disks, suggesting a surface oxide-mediated specific enhancement of fibronectin's bioactivity. Heat pretreatment had greater effects on the mRNA expression of genes for PTH-rP, alkaline phosphatase and osteocalcin while RFGD pretreatment had greater effects on osteopontin and bone sialoprotein gene expression.
CONCLUSIONS
The results suggest that heat and RFGD pretreatments of the Ti6Al4V surface oxide stimulated osteoblast differentiation through an enhancement of (a) coated fibronectin's bioactivity and (b) the bioactivities of other serum or matrix proteins. The quantitative differences in the effects of the two pretreatments on osteoblast gene marker expression may have arisen from the unique physico-chemical characteristics of each resultant oxide surface. Therefore, engineering the Ti6Al4V surface oxide to become more negatively charged can be used to accelerate osteoblast differentiation through fibronectin-dependent and independent mechanisms.
MeSH Terms
-
Alkaline Phosphatase
Alloys
Cell Differentiation
Cell Line
Collagen Type I
Dental Implants
Fees and Charges
Fibronectins
Gene Expression
Hot Temperature
Integrin alpha5beta1
Integrin-Binding Sialoprotein
Osteoblasts
Osteocalcin
Osteopontin
Parathyroid Hormone-Related Protein
Plasma
Proteins
Real-Time Polymerase Chain Reaction
RNA, Messenger
Titanium
Alkaline Phosphatase
Alloys
Collagen Type I
Dental Implants
Fibronectins
Integrin alpha5beta1
Integrin-Binding Sialoprotein
Osteocalcin
Osteopontin
Parathyroid Hormone-Related Protein
Proteins
RNA, Messenger
Titanium
Figure
Reference
-
1. Adell R, Eriksson B, Lekholm U, Branemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990. 5:347–359.2. Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981. 10:387–416.
Article3. Hardt CR, Grondahl K, Lekholm U, Wennstrom JL. Outcome of implant therapy in relation to experienced loss of periodontal bone support: a retrospective 5-year study. Clin Oral Implants Res. 2002. 13:488–494.
Article4. Grossmann Y, Levin L. Success and survival of single dental implants placed in sites of previously failed implants. J Periodontol. 2007. 78:1670–1674.
Article5. Machtei EE, Mahler D, Oettinger-Barak O, Zuabi O, Horwitz J. Dental implants placed in previously failed sites: survival rate and factors affecting the outcome. Clin Oral Implants Res. 2008. 19:259–264.
Article6. Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, et al. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996. 17:605–616.
Article7. Tengvall P, Lundstrom I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992. 9:115–134.
Article8. Morris HF, Winkler S, Ochi S. A 48-month multicentric clinical investigation: implant design and survival. J Oral Implantol. 2001. 27:180–186.
Article9. Imam MA, Fraker AC. Brown SA, Lemons JE, editors. Titanium Alloys as Implant Materials. Medical applications of titanium and its alloys. 1996. Philadelphia: American Society for Testing and Materials;3–16.
Article10. MacDonald DE, Rapuano BE, Deo N, Stranick M, Somasundaran P, Boskey AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004. 25:3135–3146.
Article11. Sousa SR, Lamghari M, Sampaio P, Moradas-Ferreira P, Barbosa MA. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J Biomed Mater Res A. 2008. 84:281–290.12. Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J Biomed Mater Res A. 2003. 64:457–464.
Article13. Rapuano BE, Wu C, MacDonald DE. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004. 22:353–361.
Article14. Sauberlich S, Klee D, Richter EJ, Hocker H, Spiekermann H. Cell culture tests for assessing the tolerance of soft tissue to variously modified titanium surfaces. Clin Oral Implants Res. 1999. 10:379–393.
Article15. MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002. 23:1269–1279.
Article16. MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 1998. 41:120–130.
Article17. Moursi AM, Damsky CH, Lull J, Zimmerman D, Doty SB, Aota S, et al. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996. 109(Pt 6):1369–1380.
Article18. Meyer U, Joos U, Mythili J, Stamm T, Hohoff A, Fillies T, et al. Ultrastructural characterization of the implant/bone interface of immediately loaded dental implants. Biomaterials. 2004. 25:1959–1967.
Article19. Hormann H. Fibronectin--mediator between cells and connective tissue. Klin Wochenschr. 1982. 60:1265–1277.20. Wagle JE, Virji AS, Williams KB, Rapley JW, MacNeill SR, Cobb CM. Can application of exogenous fibronectin enhance periodontal regeneration? J Clin Periodontol. 2002. 29:440–447.
Article21. Pearson BS, Klebe RJ, Boyan BD, Moskowicz D. Comments on the clinical application of fibronectin in dentistry. J Dent Res. 1988. 67:515–517.
Article22. Bentley KL, Klebe RJ. Fibronectin binding properties of bacteriologic petri plates and tissue culture dishes. J Biomed Mater Res. 1985. 19:757–769.
Article23. MacDonald DE, Rapuano BE, Schniepp HC. Surface oxide net charge of a titanium alloy: comparison between effects of treatment with heat or radiofrequency plasma glow discharge. Colloids Surf B Biointerfaces. 2011. 82:173–181.
Article24. Rapuano BE, MacDonald DE. Surface oxide net charge of a titanium alloy: modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf B Biointerfaces. 2011. 82:95–103.
Article25. Rapuano BE, Lee JJ, MacDonald DE. Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to α(5) β(1) integrins in osteoblasts. Eur J Oral Sci. 2012. 120:185–194.
Article26. Rapuano BE, Hackshaw KM, Schniepp HC, MacDonald DE. Surface coating of a titanium alloy with fibronectin augments expression of osteoblast gene markers in the MC3T3 osteoprogenitor cell line. J Oral Maxillofac Implants. 2012. Forthcoming.27. Erli HJ, Ruger M, Ragoss C, Jahnen-Dechent W, Hollander DA, Paar O, et al. The effect of surface modification of a porous TiO2/perlite composite on the ingrowth of bone tissue in vivo. Biomaterials. 2006. 27:1270–1276.
Article28. Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007. 148:2630–2634.
Article29. Vargo TG, Bekos EJ, Kim YS, Ranieri JP, Bellamkonda R, Aebischer P, et al. Synthesis and characterization of fluoropolymeric substrata with immobilized minimal peptide sequences for cell adhesion studies. I. J Biomed Mater Res. 1995. 29:767–778.
Article30. Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003. 66:247–259.
Article31. García AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999. 10:785–798.
Article32. Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004. 25:5947–5954.
Article33. Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007. 11:21–38.
Article34. Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005. 102:5953–5957.
Article35. Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010. 31:2728–2735.
Article36. Huang Z, Daniels RH, Enzerink RJ, Hardev V, Sahi V, Goodman SB. Effect of nanofiber-coated surfaces on the proliferation and differentiation of osteoprogenitors in vitro. Tissue Eng Part A. 2008. 14:1853–1859.
Article37. Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009. 106:2130–2135.
Article38. Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007. 28:2821–2829.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surface Characteristics and Biocompatibility of Micro Arc Oxidized Titanium Alloy
- Experimental Study of Disodium Etidronate for the Growth of MC3T3-E1 Osteoblastic Cell Line
- Gene expression of MC3T3-E1 osteoblastic cells on titanium and zirconia surface
- The biological effects of fibronectin typeIII 7-10 to MC3T3-E1 osteoblast
- Comparison of Cytocompatibility Between Grit Blasted Titanium Alloy (Ti-6Al-4V) with or without Pure Titanium Coating