J Adv Prosthodont.
2013 Nov;5(4):416-422. 10.4047/jap.2013.5.4.416.
Gene expression of MC3T3-E1 osteoblastic cells on titanium and zirconia surface
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Kyung-Hee University, Seoul, Republic of Korea. yhwoo@khu.ac.kr
- 2Department of Oral Anatomy, Dental School, Gangneung-Wonju National University, Kangnung, Republic of Korea.
- KMID: 2176532
- DOI: http://doi.org/10.4047/jap.2013.5.4.416
Abstract
- PURPOSE
This study was performed to define attachment and growth behavior of osteoblast-like cells and evaluate the gene expression on zirconia compared to titanium.
MATERIALS AND METHODS
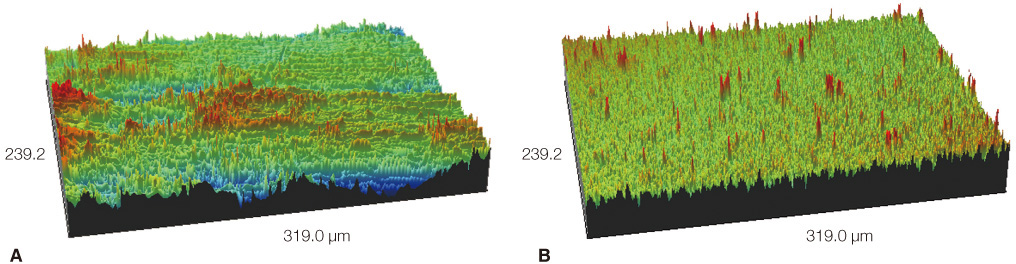
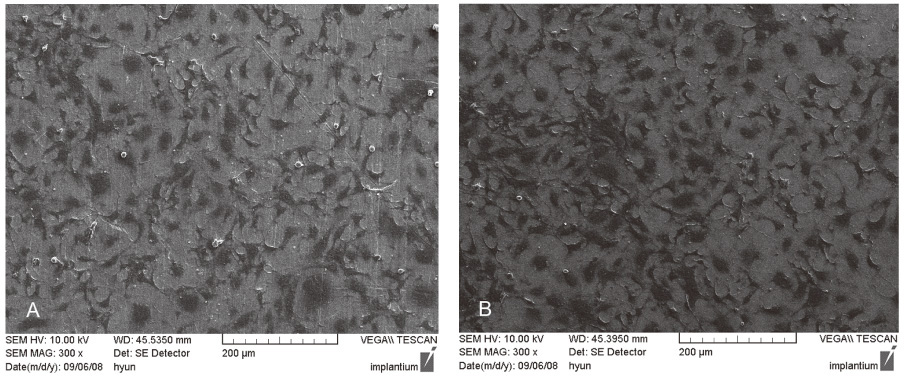
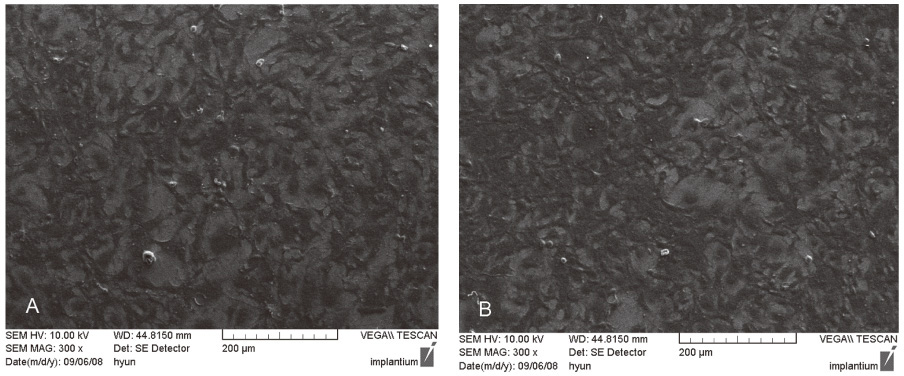
MC3T3-E1 cells were cultured on (1) titanium and (2) zirconia discs. The tetrazolium-based colorimetric assay (MTT test) was used for examining the attachment of cells. Cellular morphology was examined by scanning electron microscopy (SEM) and alkaline phosphatase (ALP) activity was measured to evaluate the cell differentiation rate. Mann-Whitney test was used to assess the significance level of the differences between the experimental groups. cDNA microarray was used for comparing the 20215 gene expressions on titanium and zirconia.
RESULTS
From the MTT assay, there was no significant difference between titanium and zirconia (P>.05). From the SEM image, after 4 hours of culture, cells on both discs were triangular or elongated in shape with formation of filopodia. After 24 hours of culture, cells on both discs were more flattened and well spread compared to 4 hours of culture. From the ALP activity assay, the optical density of E1 cells on titanium was slightly higher than that of E1 cells on zirconia but there was no significant difference (P>.05). Most of the genes related to cell adhesion showed similar expression level between titanium and zirconia.
CONCLUSION
Zirconia showed comparable biological responses of osteoblast-like cells to titanium for a short time during cell culture period. Most of the genes related to cell adhesion and signal showed similar expression level between titanium and zirconia.
MeSH Terms
Figure
Cited by 1 articles
-
Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements
Hyeongsoon Shin, Hyunjung Ko, Miri Kim
Restor Dent Endod. 2016;41(3):167-175. doi: 10.5395/rde.2016.41.3.167.
Reference
-
1. Røynesdal AK, Ambjørnsen E, Støvne S, Haanaes HR. A comparative clinical study of three different endosseous implants in edentulous mandibles. Int J Oral Maxillofac Implants. 1998; 13:500–505.2. Viornery C, Guenther HL, Aronsson BO, Péchy P, Descouts P, Grätzel M. Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res. 2002; 62:149–155.3. Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990; 5:347–359.4. van Steenberghe D. A retrospective multicenter evaluation of the survival rate of osseointegrated fixtures supporting fixed partial prostheses in the treatment of partial edentulism. J Prosthet Dent. 1989; 61:217–223.5. Schliephake H, Reiss G, Urban R, Neukam FW, Guckel S. Metal release from titanium fixtures during placement in the mandible: an experimental study. Int J Oral Maxillofac Implants. 1993; 8:502–511.6. Cortada M, Giner L, Costa S, Gil FJ, Rodríguez D, Planell JA. Galvanic corrosion behavior of titanium implants coupled to dental alloys. J Mater Sci Mater Med. 2000; 11:287–293.7. Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from titanium. Contact Dermatitis. 2000; 42:52–53.8. Henry PJ, Laney WR, Jemt T, Harris D, Krogh PH, Polizzi G, Zarb GA, Herrmann I. Osseointegrated implants for singletooth replacement: a prospective 5-year multicenter study. Int J Oral Maxillofac Implants. 1996; 11:450–455.9. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.10. Covacci V, Bruzzese N, Maccauro G, Andreassi C, Ricci GA, Piconi C, Marmo E, Burger W, Cittadini A. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999; 20:371–376.11. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798.12. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296.13. Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999; 20:2311–2321.14. Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996; 17:137–146.15. Chesmel KD, Clark CC, Brighton CT, Black J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J Biomed Mater Res. 1995; 29:1101–1110.16. Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999; 20:2311–2321.17. Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993; 69:599–604.18. Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985; 6:97–101.19. Ko HC, Han JS, Bächle M, Jang JH, Shin SW, Kim DJ. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent Mater. 2007; 23:1349–1355.20. Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994; 9:487–496.21. Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 1989; 10:104–108.22. Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992; 119:893–903.23. Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997; 9:76–85.24. Pistone M, Sanguineti C, Federici A, Sanguineti F, Defilippi P, Santolini F, Querzé G, Marchisio PC, Manduca P. Integrin synthesis and utilization in cultured human osteoblasts. Cell Biol Int. 1996; 20:471–479.25. Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000; 218:213–234.26. Jaakkola P, Jalkanen M. Transcriptional regulation of Syndecan-1 expression by growth factors. Prog Nucleic Acid Res Mol Biol. 1999; 63:109–138.27. Wongdee K, Pandaranandaka J, Teerapornpuntakit J, Tudpor K, Thongbunchoo J, Thongon N, Jantarajit W, Krishnamra N, Charoenphandhu N. Osteoblasts express claudins and tight junction-associated proteins. Histochem Cell Biol. 2008; 130:79–90.28. Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992; 262:C1119–C1124.29. Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004; 117:699–711.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response of osteoblast-like cells cultured on zirconia to bone morphogenetic protein-2
- Anti-Oral Microbial Activity and Anti-Inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Stimulated MC3T3-E1 Osteoblastic Cells on a Titanium Surface
- Comparison of alkaline phosphatase activity of MC3T3-E1 cells cultured on different Ti surfaces: modified sandblasted with large grit and acid-etched (MSLA), laser-treated, and laser and acid-treated Ti surfaces
- Cellular attachment and gene expression of osteoblast-like cells on zirconia ceramic surfaces
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1