J Bone Metab.
2013 Nov;20(2):83-88. 10.11005/jbm.2013.20.2.83.
Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Affiliations
-
- 1Department of Life and Environmental Sciences, Osaka Prefecture University, Sakai, Japan. ishibashi@biochem.osakafu-u.ac.jp
- KMID: 2286298
- DOI: http://doi.org/10.11005/jbm.2013.20.2.83
Abstract
- BACKGROUND
Estrogens exert preferable effects on bone metabolism through two estrogen receptors (ERs), ER1 and ER2, which activate the transcription of a set of genes as ligand-dependent transcription factors. Thus, growth factors and hormones which modulate ER expression in the bone, if any, may possibly modulate the effect of estrogens on bone metabolism. However, research as to which of these molecules regulate the expression of ERs in osteoblasts has not been well documented.
METHODS
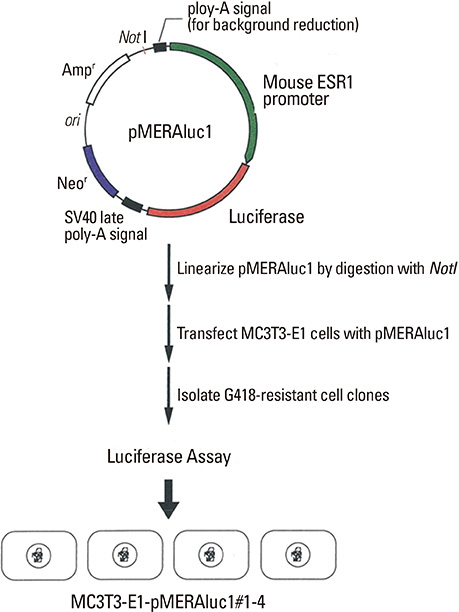
A reporter assay system developed in this study was used to explore molecules that modulate ER1 expression in MC3T3-E1 osteoblastic cells. Gene expression was analyzed by reverse transcription-polymerase chain reaction.
RESULTS
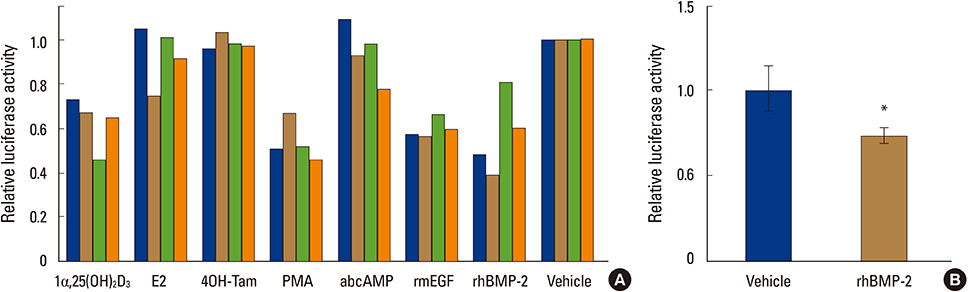
A pilot study using the reporter system revealed that bone morphogenetic protein (BMP)-2 negatively regulated ER1, but not ER2, expression in MC3T3-E1 cells. Consistently, estradiol-induced reporter activity via an estrogen responsive element was strongly suppressed in MC3T3-E1 cells pretreated with BMP-2.
CONCLUSIONS
BMP-2 desensitizes osteoblastic cells to estrogen through downregulation of ER1 expression.
MeSH Terms
-
Bone Morphogenetic Proteins
Down-Regulation*
Estrogen Receptor alpha*
Estrogens*
Gene Expression
Intercellular Signaling Peptides and Proteins
Metabolism
Osteoblasts*
Pilot Projects
Receptors, Estrogen
Transcription Factors
Bone Morphogenetic Proteins
Estrogen Receptor alpha
Estrogens
Intercellular Signaling Peptides and Proteins
Receptors, Estrogen
Transcription Factors
Figure
Reference
-
1. Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev. 1994; 15:275–300.
Article2. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012; 23:576–581.
Article3. Venken K, Callewaert F, Boonen S, et al. Sex hormones, their receptors and bone health. Osteoporos Int. 2008; 19:1517–1525.
Article4. Bland R. Steroid hormone receptor expression and action in bone. Clin Sci (Lond). 2000; 98:217–240.
Article5. Nilsson S, Gustafsson JÅ. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther. 2011; 89:44–55.
Article6. Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007; 87:905–931.
Article7. Ishibashi O, Yamagishi T, Hanada K, et al. Tamoxifen agonism and estrogen antagonism of c-fos gene promoter activity through non-consensus-responsive elements in MC3T3-E1 osteoblasts. Biochem Biophys Res Commun. 2001; 289:705–711.
Article8. Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003; 125:143–149.
Article9. Emmen JM, Korach KS. Estrogen receptor knockout mice: phenotypes in the female reproductive tract. Gynecol Endocrinol. 2003; 17:169–176.
Article10. McCauley LK, Tözüm TF, Rosol TJ. Estrogen receptors in skeletal metabolism: lessons from genetically modified models of receptor function. Crit Rev Eukaryot Gene Expr. 2002; 12:89–100.
Article11. Hawse JR, Subramaniam M, Ingle JN, et al. Estrogen-TGFbeta cross-talk in bone and other cell types: role of TIEG, Runx2, and other transcription factors. J Cell Biochem. 2008; 103:383–392.
Article12. Matsuda T, Yamamoto T, Muraguchi A, et al. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001; 276:42908–42914.
Article13. Wu L, Wu Y, Gathings B, et al. Smad4 as a transcription corepressor for estrogen receptor alpha. J Biol Chem. 2003; 278:15192–15200.14. Yamamoto T, Saatcioglu F, Matsuda T. Cross-talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology. 2002; 143:2635–2642.
Article15. Yamamoto T, Matsuda T, Junicho A, et al. Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett. 2000; 486:143–148.
Article16. Ishibashi O, Kawashima H. Cloning and characterization of the functional promoter of mouse estrogen receptor beta gene. Biochim Biophys Acta. 2001; 1519:223–229.
Article17. Kato S, Masuhiro Y, Watanabe M, et al. Molecular mechanism of a cross-talk between oestrogen and growth factor signalling pathways. Genes Cells. 2000; 5:593–601.
Article18. Stoica A, Saceda M, Fakhro A, et al. Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem. 1999; 75:640–651.
Article19. Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000; 6:3371–3379.20. Lonard DM, Nawaz Z, Smith CL, et al. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000; 5:939–948.
Article21. Reid G, Hübner MR, Métivier R, et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003; 11:695–707.
Article22. Ihionkhan CE, Chambliss KL, Gibson LL, et al. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002; 91:814–820.
Article23. Tschugguel W, Dietrich W, Zhegu Z, et al. Differential regulation of proteasome-dependent estrogen receptor alpha and beta turnover in cultured human uterine artery endothelial cells. J Clin Endocrinol Metab. 2003; 88:2281–2287.
Article24. Matsumoto Y, Otsuka F, Takano-Narazaki M, et al. Estrogen facilitates osteoblast differentiation by upregulating bone morphogenetic protein-4 signaling. Steroids. 2013; 78:513–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells
- Effect of Estrogen on the Tumor Necrosis Factor-alpha-induced Apoptosis and Cytokine Gene Expression in MC3T3-E1 Osteoblast
- Effects of estrogen receptor and estrogen on the chromatin structure in estrogen receptor stable transfectants
- On the Activity of MC3T3-E1 Cell in vitro
- Molecular mechanisms of hederagenin in bone formation