J Adv Prosthodont.
2016 Jun;8(3):235-240. 10.4047/jap.2016.8.3.235.
Comparison of alkaline phosphatase activity of MC3T3-E1 cells cultured on different Ti surfaces: modified sandblasted with large grit and acid-etched (MSLA), laser-treated, and laser and acid-treated Ti surfaces
- Affiliations
-
- 1Department of Prosthodontics, College of Dentistry, Kyungpook National University, Daegu, Republic of Korea. sacho@knu.ac.kr
- 2CSM IMPLANT Surface Treatment Institute, Daegu, Republic of Korea.
- KMID: 2376850
- DOI: http://doi.org/10.4047/jap.2016.8.3.235
Abstract
- PURPOSE
In this study, the aim of this study was to evaluate the effect of implant surface treatment on cell differentiation of osteoblast cells. For this purpose, three surfaces were compared: (1) a modified SLA (MSLA: sand-blasted with large grit, acid-etched, and immersed in 0.9% NaCl), (2) a laser treatment (LT: laser treatment) titanium surface and (3) a laser and acid-treated (LAT: laser treatment, acid-etched) titanium surface.
MATERIALS AND METHODS
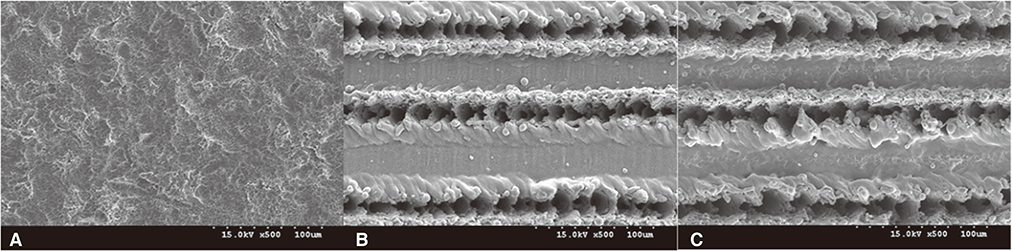
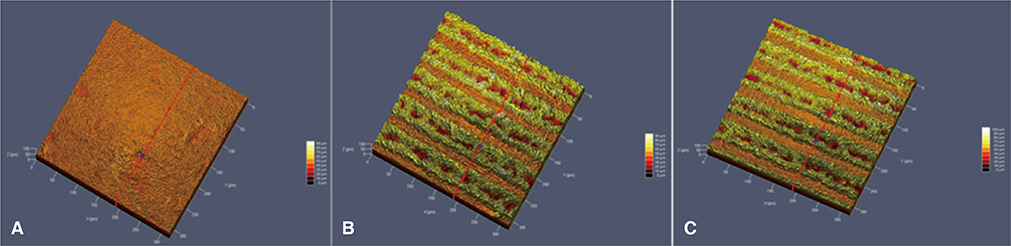
The MSLA surfaces were considered as the control group, and LT and LAT surfaces as test groups. Alkaline phosphatase expression (ALP) was used to quantify osteoblastic differentiation of MC3T3-E1 cell. Surface roughness was evaluated by a contact profilometer (URFPAK-SV; Mitutoyo, Kawasaki, Japan) and characterized by two parameters: mean roughness (Ra) and maximum peak-to-valley height (Rt).
RESULTS
Scanning electron microscope revealed that MSLA (control group) surface was not as rough as LT, LAT surface (test groups). Alkaline phosphatase expression, the measure of osteoblastic differentiation, and total ALP expression by surface-adherent cells were found to be highest at 21 days for all three surfaces tested (P<.05). Furthermore, ALP expression levels of MSLA and LAT surfaces were significantly higher than expression levels of LT surface-adherent cells at 7, 14, and 21 days, respectively (P<.05). However, ALP expression levels between MSLA and LAT surface were equal at 7, 14, and 21 days (P>.05).
CONCLUSION
This study suggested that MSLA and LAT surfaces exhibited more favorable environment for osteoblast differentiation when compared with LT surface, the results that are important for implant surface modification studies.
Figure
Reference
-
1. Sammons RL, Lumbikanonda N, Gross M, Cantzler P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. A scanning electron microscopic study. Clin Oral Implants Res. 2005; 16:657–666.2. Zhang F, Yang GL, He FM, Zhang LJ, Zhao SF. Cell response of titanium implant with a roughened surface containing titanium hydride: an in vitro study. J Oral Maxillofac Surg. 2010; 68:1131–1139.3. Alfarsi MA, Hamlet SM, Ivanovski S. Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response. J Biomed Mater Res A. 2014; 102:60–67.4. Gu YX, Du J, Si MS, Mo JJ, Qiao SC, Lai HC. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J Biomed Mater Res A. 2013; 101:748–754.5. Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2010; 93:289–296.6. Bang SM, Moon HJ, Kwon YD, Yoo JY, Pae A, Kwon IK. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin Oral Implants Res. 2014; 25:831–837.7. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–334.8. Forsgren J, Paz MD, León B, Engqvist H. Laser induced surface structuring and ion conversion in the surface oxide of titanium: possible implications for the wetability of laser treated implants. J Mater Sci Mater Med. 2013; 24:11–15.9. Rong M, Zhou L, Gou Z, Zhu A, Zhou D. The early osseointegration of the laser-treated and acid-etched dental implants surface: an experimental study in rabbits. J Mater Sci Mater Med. 2009; 20:1721–1728.10. Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K. Determining optimal dose of laser therapy for attachment and proliferation of human oral fibroblasts cultured on titanium implant material. J Biomed Mater Res A. 2005; 73:55–62.11. Khadra M, Kasem N, Lyngstadaas SP, Haanaes HR, Mustafa K. Laser therapy accelerates initial attachment and subsequent behaviour of human oral fibroblasts cultured on titanium implant material. A scanning electron microscope and histomorphometric analysis. Clin Oral Implants Res. 2005; 16:168–175.12. Zhang EW, Wang YB, Shuai KG, Gao F, Bai YJ, Cheng Y, Xiong XL, Zheng YF, Wei SC. In vitro and in vivo evaluation of SLA titanium surfaces with further alkali or hydrogen peroxide and heat treatment. Biomed Mater. 2011; 6:025001.13. Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990; 143:420–430.14. Chen WC, Chen YS, Ko CL, Lin Y, Kuo TH, Kuo HN. Interaction of progenitor bone cells with different surface modifications of titanium implant. Mater Sci Eng C Mater Biol Appl. 2014; 37:305–313.15. Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011; 26:1256–1266.16. Le Guehennec L, Lopez-Heredia MA, Enkel B, Weiss P, Amouriq Y, Layrolle P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008; 4:535–543.17. Hughes FJ, Collyer J, Stanfield M, Goodman SA. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology. 1995; 136:2671–2677.18. Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998; 19:2219–2232.19. Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001; 4:3–14.20. Cho SA, Jung SK. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials. 2003; 24:4859–4863.21. Chen WC, Lo Y, Chen HS. Effects of Ti surface treatments with silane and arginylglycylaspartic acid peptide on bone cell progenitors. Odontology. 2015; 103:322–332.22. Souza FA, Queiroz TP, Guastaldi AC, Garcia-Júnior IR, Magro-Filho O, Nishioka RS, Sisti KE, Sonoda CK. Comparative in vivo study of commercially pure Ti implants with surfaces modified by laser with and without silicate deposition: biomechanical and scanning electron microscopy analysis. J Biomed Mater Res B Appl Biomater. 2013; 101:76–84.23. Györgyey Á, Ungvári K, Kecskeméti G, Kopniczky J, Hopp B, Oszkó A, Pelösczi I, Rakonczay Z, Nagy K, Turzó K. Attachment and proliferation of human osteoblast-like cells (MG-63) on laser-ablated titanium implant material. Mater Sci Eng C Mater Biol Appl. 2013; 33:4251–4259.24. Guo Z, Zhou L, Rong M, Ding J, Zhu A, Li S, Lu H. Bone augmentation in a titanium cap with a porous surface modified by microarc oxidation. Int J Oral Maxillofac Implants. 2013; 28:767–773.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of removal torques between laser-etched and modified sandblasted acid-etched Ti implant surfaces in rabbit tibias
- Hydroxyapatite Nanorod-Modified Sand Blasted Titanium Disk for Endosseous Dental Implant Applications
- Effect of Rosmarinic Acid on the Focal Adhesions of MC3T3-E1 Preosteoblasts on Titanium Surface
- Effect of etched microgrooves on hydrophilicity of titanium and osteoblast responses: A pilot study
- Scanning Electron Microscopic Study of the Effects of Citric Acid on the Change of Implant Surface According to Application Time