Korean J Physiol Pharmacol.
2009 Aug;13(4):301-307. 10.4196/kjpp.2009.13.4.301.
DHA and EPA Down-regulate COX-2 Expression through Suppression of NF-kappa B Activity in LPS-treated Human Umbilical Vein Endothelial Cells
- Affiliations
-
- 1Department of Obstetrics & Gynecology, School of Medicine, Gyeongsang National University, Jinju 660-702, Korea. wypaik@gnu.ac.kr
- 2Institute of Health Sciences, School of Medicine, Gyeongsang National University, Jinju 660-702, Korea.
- 3Department of Pharmacology, School of Medicine, Gyeongsang National University, Jinju 660-702, Korea.
- KMID: 2071685
- DOI: http://doi.org/10.4196/kjpp.2009.13.4.301
Abstract
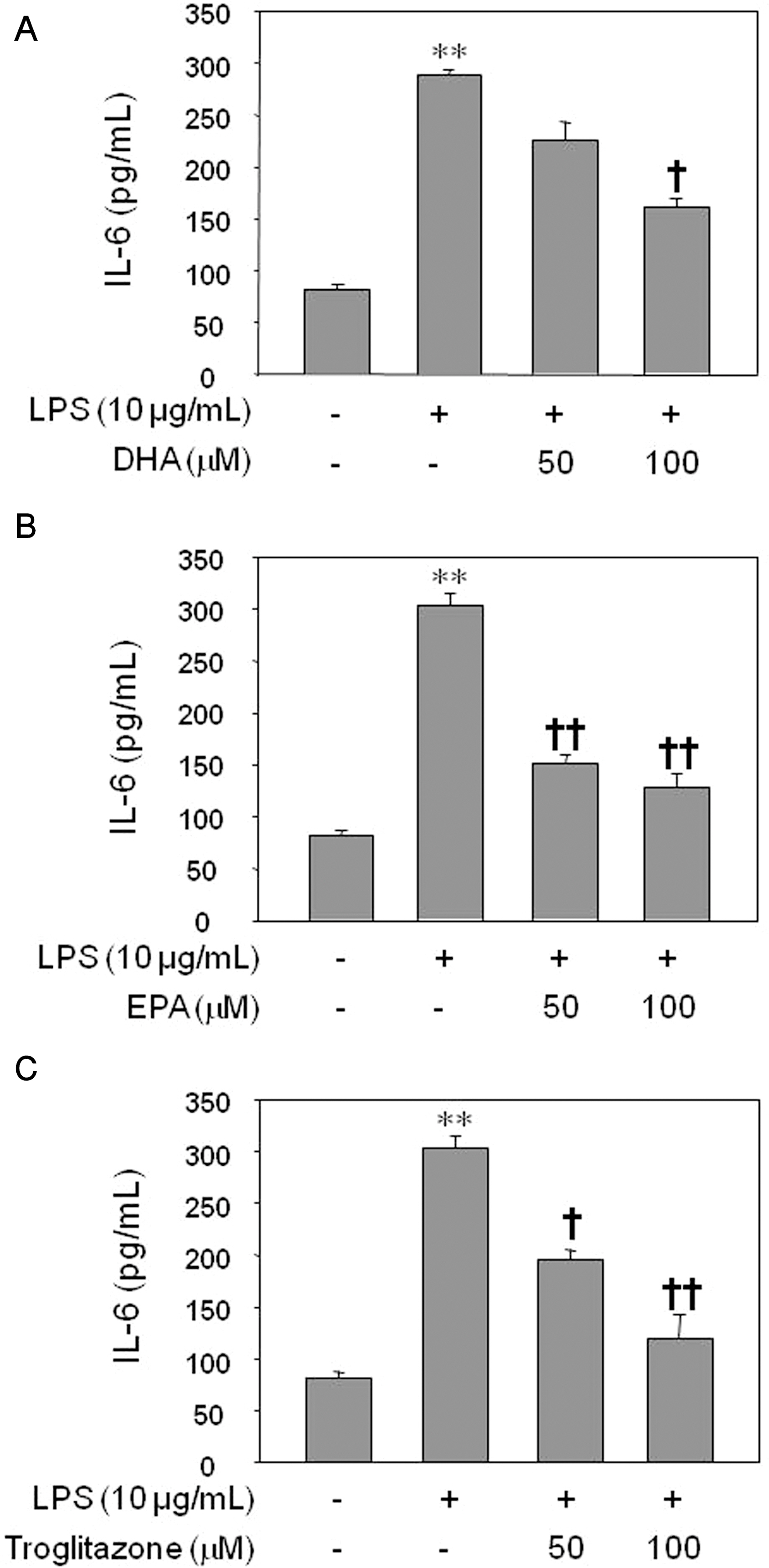
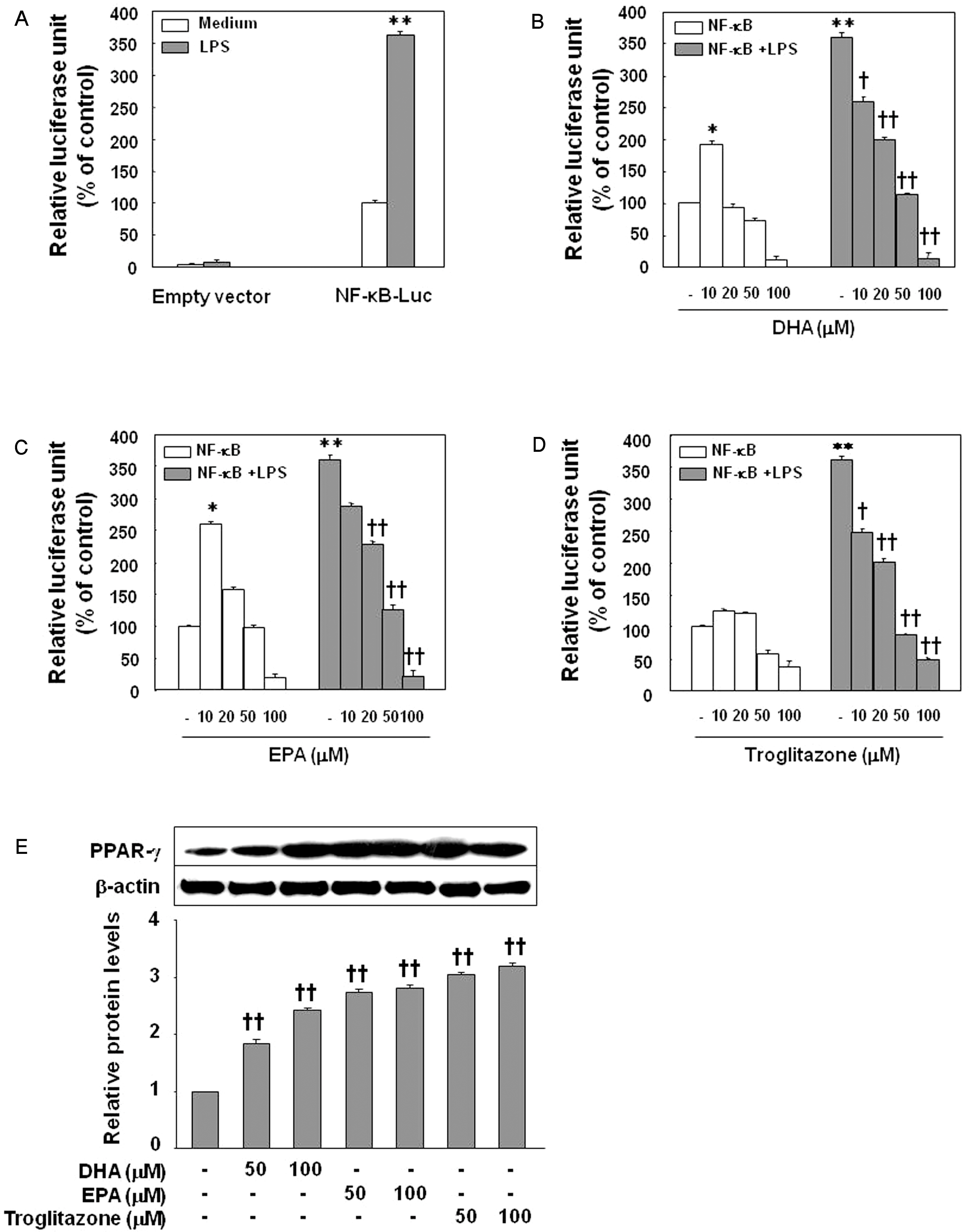
- Inflammatory processes of vascular endothelial cells play a key role in the development ofatherosclerosis. We determined the anti-inflammatory effects and mechanisms of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on LPS-treated human umbilical vein endothelial cells (HUVECs) to evaluate their cardioprotective potential. Cells were pretreated with DHA, EPA, or troglitazone prior to activation with LPS. Expression of COX-2, prostaglandin E2 (PGE2) and IL-6 production, and NF-kappaB activity were measured by Western blot, ELISA, and luciferase activity, respectively. Results showed that EPA, DHA, or troglitazone significantly reduced COX-2 expression, NF-kappaB luciferase activity, and PGE2 and IL-6 production in a dose-dependent fashion. Interestingly, low doses (10 micrometer) of DHA and EPA, but not troglitozone, significantly increased the activity of NF-kappaB in resting HUVECs. Our study suggests that while DHA, EPA, and troglitazone may be protective on HUVECs under inflammatory conditions in a dose-dependent manner. However there may be some negative effects when the concentrations are abnormally low, even in normal endothelium.
Keyword
MeSH Terms
-
Blotting, Western
Chromans
Cyclooxygenase 2
Dinoprostone
Eicosapentaenoic Acid
Endothelial Cells
Endothelium
Enzyme-Linked Immunosorbent Assay
Human Umbilical Vein Endothelial Cells
Humans
Interleukin-6
Luciferases
NF-kappa B
Thiazolidinediones
Chromans
Cyclooxygenase 2
Dinoprostone
Eicosapentaenoic Acid
Interleukin-6
Luciferases
NF-kappa B
Thiazolidinediones
Figure
Reference
-
Abeywardena MY., Head RJ. Long chain n-3 polyunsaturatedfatty acids and blood vessel function. Cardiovasc Res. 52:361–371. 2001.Bagga D., Wang L., Farias-Eisner R., Glaspy JA., Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 100:1751–1756. 2003.Blaschke F., Spanheimer R., Khan M., Law RE. Vascular effects of TZDs: New implications. Vascul Pharmacol. 45:3–18. 2006.
ArticleCipollone F., Fazia ML. Cyclooxygenase-2 inhibition: vascular inflammation and cardiovascular risk. Curr Atheroscler Rep. 8:245–251. 2006.
ArticleDelerive P., Fruchart JC., Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 169:453–459. 2001.
ArticleEngler MB., Engler MM., Browne A., Sun YP., Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 131:1793–1799. 2000.
ArticleGoua M., Mulgrew S., Frank J., Rees D., Sneddon AA., Wahle KW. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: involvement of the transcription factor NF-kappaB? Prostaglandins Leukot Essent Fatty Acids. 78:33–43. 2008.Hayden MS., Ghosh S. Signaling to NF-κB. Genes Dev. 18:2195–2224. 2004.
ArticleKazemi MR., McDonald CM., Shigenaga JK., Grunfeld C., Feingold KR. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. 25:1220–1224. 2005.
ArticleKim HJ., Tsoy I., Park JM., Chung JI., Shin SC., Chang KC. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 580:1391–1397. 2006.Kris-Etherton PM., Harris WS., Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 106:2747–2757. 2002.
ArticleLibby CP. Inflammation in atherosclerosis. Nature. 420:868–874. 2002.
ArticleMassaro M., Habib A., Lubrano L., Del Turco S., Lazzerini G., Bourcier T., Weksler BB., De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP (H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 103:15184–15189. 2006.Massaro M., Scoditti E., Carluccio MA., De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 79:109–115. 2008.
ArticlePhilip CC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83(Suppl):1505S–1519S. 2006.Rosen ED., Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 276:37731–37734. 2001.
ArticleRoss R. Atherosclerosis – an inflammatory disease. N Engl J Med. 340:115–126. 1999.
ArticleScher JU., Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009. Feb 20. [Epub ahead of print].
ArticleSerhan CN., Clish CB., Brannon J., Colgan SP., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 192:1197–1204. 2000.Serhan CN., Hong S., Gronert K., Colgan SP., Devchand PR., Mirick G., Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 196:1025–1037. 2002.Stary HC., Chandler AB., Glagov S., Guyton JR., Insull W Jr., Rosenfeld ME., Schaffer SA., Schwartz CJ., Wagner WD., Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation. 89:2462–2478. 1994.
ArticleStoll LL., Denning GM., Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 24:2227–2236. 2004.
ArticleTriantafilou M., Gamper FG., Lepper PM., Mouratis MA., Schumann C., Harokopakis E., Schifferle RE., Hajishengallis G., Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 9:2030–2039. 2007.
ArticleUmetani M., Mataki C., Minegishi N., Yamamoto M., Hamakubo T., Kodama T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 21:917–922. 2001.Vikatmaa P., Lajunen T., Ikonen TS., Pussinen PJ., Lepäntalo M., Leinonen M., Saikku P. Chlamydial lipopolysaccharide (cLPS) is present in atherosclerotic and aneurysmal arterial wall-cLPS levels depend on disease manifestation. Cardiovasc Pathol. 2009. Jan 14. [Epub ahead of print].
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Eicosapentaenoic Acid on Endothelial Cell-U937 Cell Adhesion
- Activation of Nuclear Factor Kappa B by Inducers in Gestational Tissues at Term
- GPR40 Agonism Modulates Inflammatory Reactions in Vascular Endothelial Cells
- Pro-inflammatory cytokine-induced nuclear factor-kappa B activity in prelabor and in-labor myometrial cells at term gestation
- Ginsenoside Rg2 Inhibits Lipopolysaccharide-Induced Adhesion Molecule Expression in Human Umbilical Vein Endothelial Cell