Clin Exp Vaccine Res.
2015 Jan;4(1):59-67. 10.7774/cevr.2015.4.1.59.
Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine
- Affiliations
-
- 1Clinical Vaccine R&D Center, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea. jhrhee@chonnam.ac.kr
- 2Department of Pharmacology and Dental Therapeutics, School of Dentistry, Chonnam National University, Gwangju, Korea.
- 3Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2049108
- DOI: http://doi.org/10.7774/cevr.2015.4.1.59
Abstract
- PURPOSE
Recombinant subunit vaccines provide safe and targeted protection against microbial infections. However, the protective efficacy of recombinant subunit vaccines tends to be less potent than the whole cell vaccines, especially when they are administered through mucosal routes. We have reported that a bacterial flagellin has strong mucosal adjuvant activity to induce protective immune responses. In this study, we tested whether FlaB could be used as a fusion partner of subunit vaccine for tetanus.
MATERIALS AND METHODS
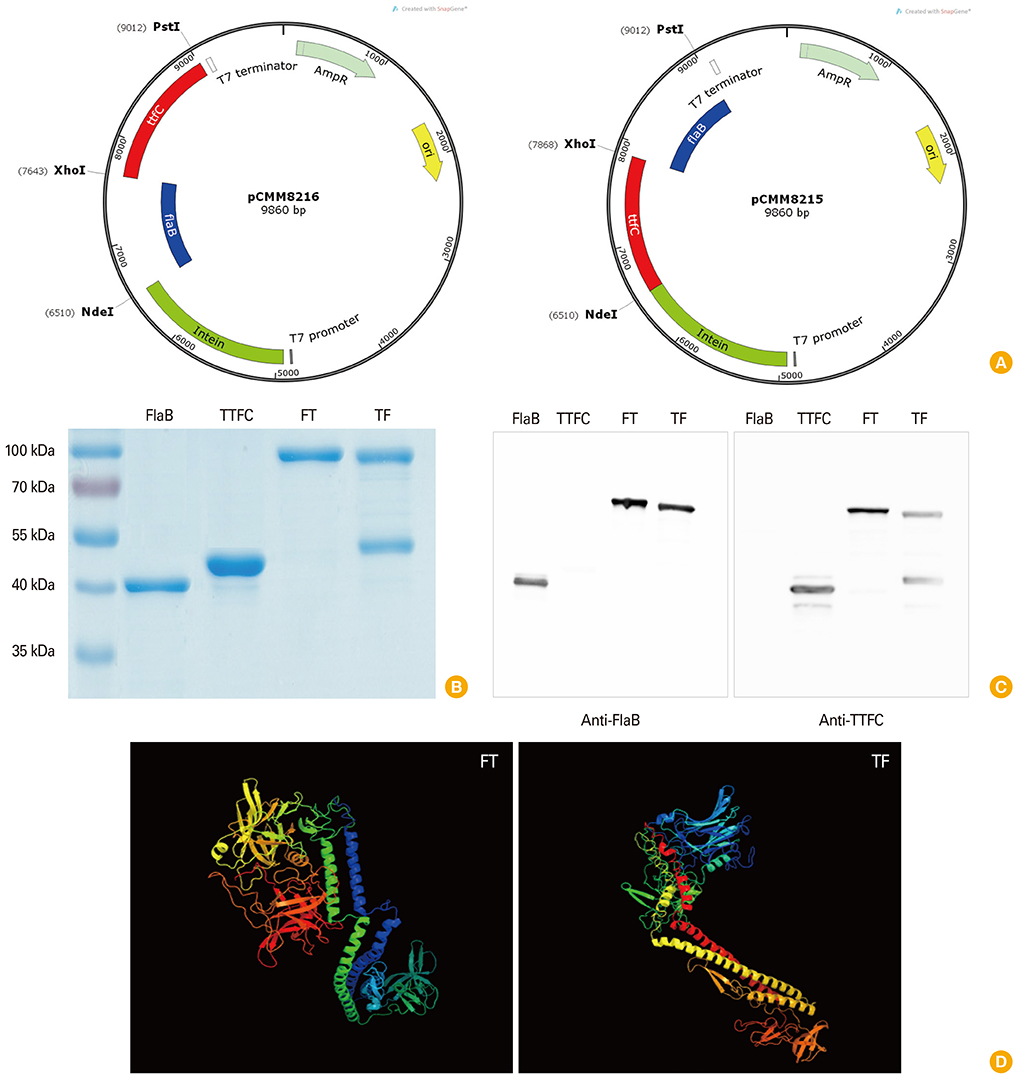
We constructed fusion proteins consisted with tetanus toxin fragment C (TTFC), the nontoxic C-terminal portion of tetanus toxin, and a Toll-like receptor 5 agonist from Vibrio vulnificus (FlaB). Mice were intranasally administered with fusion protein and protective immune responses of the vaccinated mice were analyzed.
RESULTS
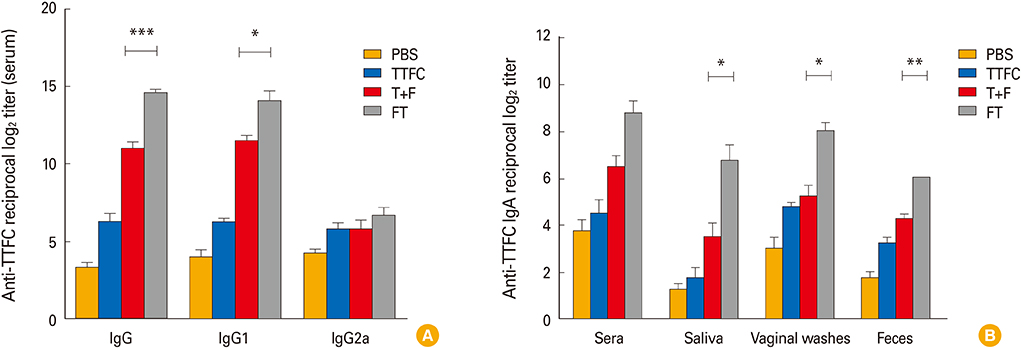
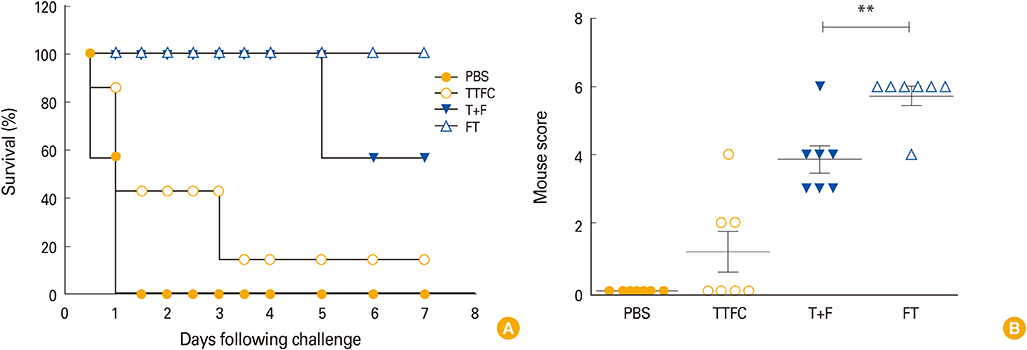
FlaB-TTFC recombinant protein induced strong tetanus-specific antibody responses in both systemic and mucosal compartments and prolonged the survival of mice after challenge with a supra-lethal dose of tetanus toxin.
CONCLUSION
This study establishes FlaB as a successful fusion partner for recombinant subunit tetanus vaccine applicable through mucosal route, and it further endorses our previous observations that FlaB could be a stable adjuvant partner for mucosal vaccines.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Fusion Protein of Derp2 Allergen and Flagellin Suppresses Experimental Allergic Asthma
Wenzhi Tan, Jin Hai Zheng, Tra-My Nu Duong, Young-Il Koh, Shee Eun Lee, Joon Haeng Rhee
Allergy Asthma Immunol Res. 2019;11(2):254-266. doi: 10.4168/aair.2019.11.2.254.
Reference
-
1. Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011; 9:889–893.
Article2. Unnikrishnan M, Rappuoli R, Serruto D. Recombinant bacterial vaccines. Curr Opin Immunol. 2012; 24:337–342.
Article3. Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin. 2007; 3:205–211.
Article4. Brown BK, Cox J, Gillis A, et al. Phase I study of safety and immunogenicity of an Escherichia coli-derived recombinant protective antigen (rPA) vaccine to prevent anthrax in adults. PLoS One. 2010; 5:e13849.
Article5. Pizza M, Covacci A, Bartoloni A, et al. Mutants of pertussis toxin suitable for vaccine development. Science. 1989; 246:497–500.
Article6. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013; 19:1597–1608.
Article7. Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012; 1:50–63.
Article8. Riese P, Schulze K, Ebensen T, Prochnow B, Guzman CA. Vaccine adjuvants: key tools for innovative vaccine design. Curr Top Med Chem. 2013; 13:2562–2580.
Article9. Lee SE, Kim SY, Jeong BC, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006; 74:694–702.
Article10. Perez O, Romeu B, Cabrera O, et al. Adjuvants are Key Factors for the Development of Future Vaccines: Lessons from the Finlay Adjuvant Platform. Front Immunol. 2013; 4:407.11. Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010; 28:Suppl 3. C25–C36.12. Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–1103.
Article13. Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006; 7:868–874.
Article14. Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine. 2011; 29:5731–5739.
Article15. Hong SH, Byun YH, Nguyen CT, et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012; 30:466–474.
Article16. Kim SY, Thanh XT, Jeong K, et al. Contribution of six flagellin genes to the flagellum biogenesis of Vibrio vulnificus and in vivo invasion. Infect Immun. 2014; 82:29–42.
Article17. Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013; 8:360–376.
Article18. Foged C. Subunit vaccines of the future: the need for safe, customized and optimized particulate delivery systems. Ther Deliv. 2011; 2:1057–1077.
Article19. Kaufmann SH. Tuberculosis vaccine development at a divide. Curr Opin Pulm Med. 2014; 20:294–300.
Article20. Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001; 87:477–487.
Article21. Ribas AV, Ho PL, Tanizaki MM, Raw I, Nascimento AL. High-level expression of tetanus toxin fragment C-thioredoxin fusion protein in Escherichia coli. Biotechnol Appl Biochem. 2000; 31(Pt 2):91–94.
Article22. Norton PM, Wells JM, Brown HW, Macpherson AM, Le Page RW. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactis expressing tetanus toxin fragment C. Vaccine. 1997; 15:616–619.
Article23. Abreu PA, Miyasato PA, Vilar MM, et al. Sm14 of Schistosoma mansoni in fusion with tetanus toxin fragment C induces immunoprotection against tetanus and schistosomiasis in mice. Infect Immun. 2004; 72:5931–5937.
Article24. Jang JI, Kim JS, Eom JS, et al. Expression and delivery of tetanus toxin fragment C fused to the N-terminal domain of SipB enhances specific immune responses in mice. Microbiol Immunol. 2012; 56:595–604.
Article25. Amuguni JH, Lee S, Kerstein KO, et al. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine. 2011; 29:4778–4784.
Article26. Makoff AJ, Oxer MD, Romanos MA, Fairweather NF, Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989; 17:10191–10202.
Article27. Curtiss RI, Zhang X, Wanda SY, et al. Induction of host immune responses using Salmonella-vectored vaccines. In : Brogden KA, Minion FC, Cornick N, editors. Virulence mechanisms of bacterial pathogens. 4th ed. Washington, DC: ASM Press;2007. p. 297–313.28. Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009; 4:363–371.
Article29. Ipsen J Jr. The effect of environmental temperature on the immune response of mice to tetanus toxoid. J Immunol. 1952; 69:273–283.30. Junqueira-Kipnis AP, Marques Neto LM, Kipnis A. Role of fused Mycobacterium tuberculosis immunogens and adjuvants in modern tuberculosis vaccines. Front Immunol. 2014; 5:188.
Article31. Smith KD, Andersen-Nissen E, Hayashi F, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003; 4:1247–1253.
Article32. Bartoloni A, Norelli F, Ceccarini C, Rappuoli R, Costantino P. Immunogenicity of meningococcal B polysaccharide conjugated to tetanus toxoid or CRM197 via adipic acid dihydrazide. Vaccine. 1995; 13:463–470.
Article33. Bargieri DY, Soares IS, Costa FT, Braga CJ, Ferreira LC, Rodrigues MM. Malaria vaccine development: are bacterial flagellin fusion proteins the bridge between mouse and humans? J Parasitol Res. 2011; 2011:965369.
Article34. Savar NS, Jahanian-Najafabadi A, Mahdavi M, Shokrgozar MA, Jafari A, Bouzari S. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J Biotechnol. 2014; 175:31–37.
Article35. Wang BZ, Gill HS, He C, et al. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release. 2014; 178:1–7.
Article36. Girard A, Roques E, Massie B, Archambault D. Flagellin in fusion with human rotavirus structural proteins exerts an adjuvant effect when delivered with replicating but non-disseminating adenovectors through the intrarectal route. Mol Biotechnol. 2014; 56:394–407.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of the Heat Shock Protein 70 on the Adjuvanticity Induced by a Bacterial Flagellin of Vibrio ulnificus
- Evaluation of Potency on Diphtheria and Tetanus Toxoid for Adult Vaccines by In Vivo Toxin Neutralization Assay Using National Reference Standards
- Rates of Adverse Reactions Associated with Modified DPT Vaccine in Korean Infants and Children
- Bacterial flagellin—a potent immunomodulatory agent
- Antltoxln response to diphtheria and tetananus vaccine