Anat Cell Biol.
2013 Dec;46(4):262-271. 10.5115/acb.2013.46.4.262.
Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression
- Affiliations
-
- 1Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea. hyi830@snu.ac.kr
- 2Department of Physical and Rehabilitation Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2046767
- DOI: http://doi.org/10.5115/acb.2013.46.4.262
Abstract
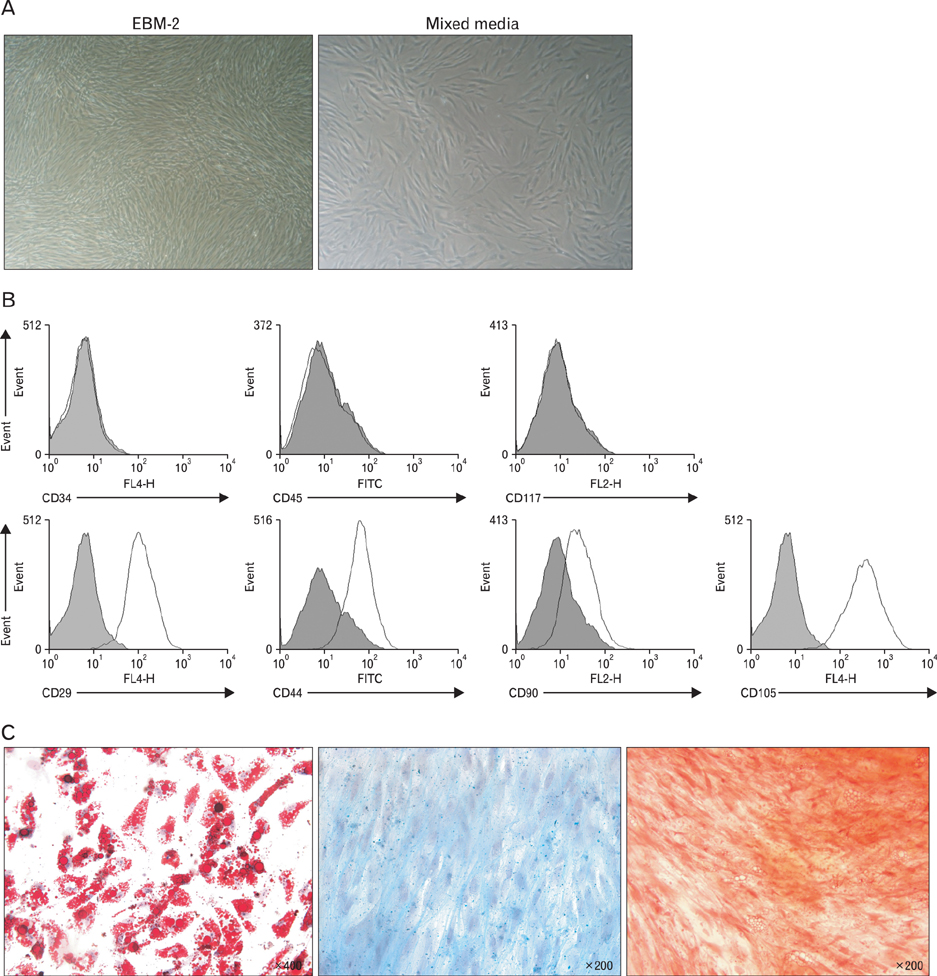
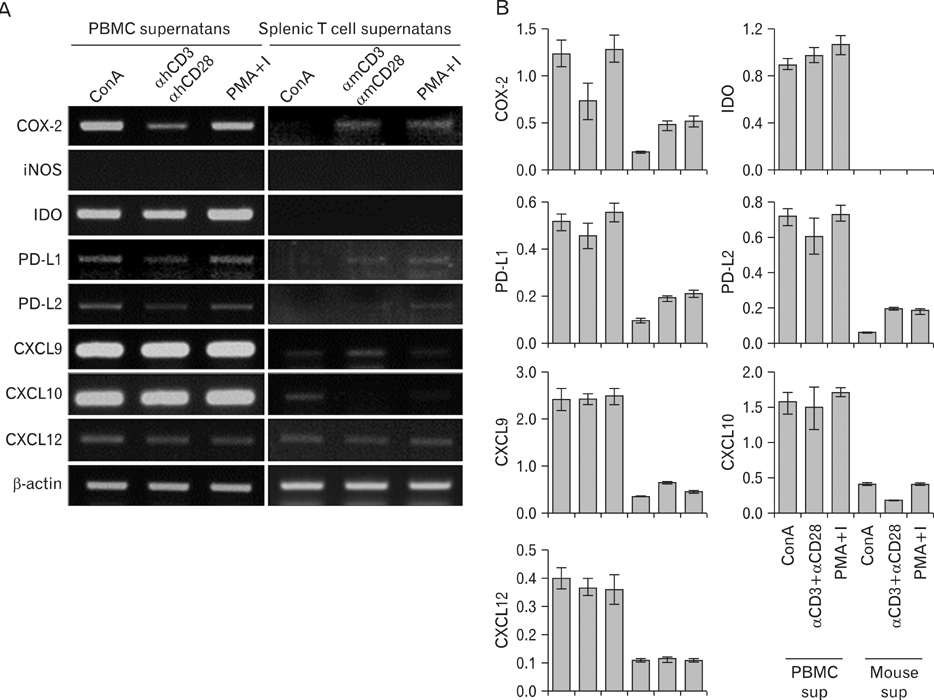
- Mesenchymal stem cells (MSCs) of human origin have been frequently applied to experimental animal models to evaluate their immunomodulatory functions. MSCs are known to be activated by cytokines from T cells, predominantly by interferon-gamma (IFN-gamma), in conjunction with other cytokines such as tumor necrosis factor-alpha (TNF-alpha) and interlukin-1beta. Because IFN-gamma is not cross-reactive between human and mouse species, the manner in which human MSCs administered in experimental animals are activated and stimulated to function has been questioned. In the present study, we established MSCs from human adipose tissue. They successfully suppressed the proliferation of not only human peripheral blood mononuclear cells but also mouse splenic T cells. When these human MSCs were stimulated with a culture supernatant of mouse T cells or recombinant murine TNF-alpha, they expressed cyclooxygenase-2 (COX-2), but not indoleamine 2,3-dioxygenase. The dominant role of COX-2 in suppressing mouse T cell proliferation was validated by the addition of COX-2 inhibitor in the co-culture, wherein the suppressed proliferation was almost completely recovered. In conclusion, human MSCs in a murine environment were activated, at least in part, by TNF-alpha and mainly used COX-2 as a tool for the suppression of in vitro T cell proliferation. These results should be considered when interpreting results for human MSCs in experimental animals.
MeSH Terms
-
Adipose Tissue
Animals
Cell Proliferation*
Coculture Techniques
Cyclooxygenase 2*
Cytokines
Humans*
Immunomodulation
Indoleamine-Pyrrole 2,3,-Dioxygenase
Interferon-gamma
Mesenchymal Stromal Cells*
Mice
Models, Animal
T-Lymphocytes
Tumor Necrosis Factor-alpha
Cyclooxygenase 2
Cytokines
Indoleamine-Pyrrole 2,3,-Dioxygenase
Interferon-gamma
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011; 2:14.2. Stoltz JF, Huselstein C, Schiavi J, Li YY, Bensoussan D, Decot V, De Isla N. Human stem cells and articular cartilage tissue engineering. Curr Pharm Biotechnol. 2012; 13:2682–2691.3. De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012; 12:574–591.4. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012; 18:128–134.5. DelaRosa O, Lombardo E, Beraza A, Mancheño-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, García L, Abad JL, Trigueros C, Delgado M, Büscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009; 15:2795–2806.6. English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007; 110:91–100.7. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2:141–150.8. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008; 17:681–693.9. Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004; 103:4619–4621.10. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109:228–234.11. Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008; 18:846–857.12. Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007; 17:240–248.13. Ren G, Su J, Zhang L, Zhao X, Ling W, L'Huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009; 27:1954–1962.14. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–1441.15. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009; 136:978–989.16. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009; 60:1006–1019.17. Zhou B, Yuan J, Zhou Y, Ghawji M Jr, Deng YP, Lee AJ, Nair U, Kang AH, Brand DD, Yoo TJ. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011; 141:328–337.18. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006; 103:17438–17443.19. Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007; 2:e941.20. Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008; 31:395–405.21. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008; 107:141–151.22. Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, Zeng X, Shi S, Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010; 19:1502–1514.23. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.24. Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008; 10:784–795.25. Hemmi S, Peghini P, Metzler M, Merlin G, Dembic Z, Aguet M. Cloning of murine interferon gamma receptor cDNA: expression in human cells mediates high-affinity binding but is not sufficient to confer sensitivity to murine interferon gamma. Proc Natl Acad Sci U S A. 1989; 86:9901–9905.26. Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007; 149:353–363.27. Linscheid P, Seboek D, Zulewski H, Scherberich A, Blau N, Keller U, Müller B. Cytokine-induced metabolic effects in human adipocytes are independent of endogenous nitric oxide. Am J Physiol Endocrinol Metab. 2006; 290:E1068–E1077.28. Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000; 157:729–735.29. Usui Y, Okunuki Y, Hattori T, Takeuchi M, Kezuka T, Goto H, Usui M. Expression of costimulatory molecules on human retinoblastoma cells Y-79: functional expression of CD40 and B7H1. Invest Ophthalmol Vis Sci. 2006; 47:4607–4613.30. Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clin Exp Immunol. 2007; 149:317–326.31. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.32. Gieseke F, Schütt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007; 110:2197–2200.33. Lembo D, Ricciardi-Castagnoli P, Alber G, Ozmen L, Landolfo S, Blüthmann H, Dembic Z, Kotenko SV, Cook JR, Pestka S, Garotta G. Mouse macrophages carrying both subunits of the human interferon-gamma (IFN-gamma) receptor respond to human IFN-gamma but do not acquire full protection against viral cytopathic effect. J Biol Chem. 1996; 271:32659–32666.34. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–1822.35. Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006; 281:13964–13971.36. Fransen L, Ruysschaert MR, Van der Heyden J, Fiers W. Recombinant tumor necrosis factor: species specificity for a variety of human and murine transformed cell lines. Cell Immunol. 1986; 100:260–267.37. Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, Zago MA, Panepucci RA. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011; 7:66–74.38. Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012; 10:544–555.39. Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010; 15:411–415.40. Scheerlinck JP. Functional and structural comparison of cytokines in different species. Vet Immunol Immunopathol. 1999; 72:39–44.41. Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013; 65:1181–1193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose-derived stem cells: characterization and clinical application
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Adipose Tissue Derived Mesenchymal Stem Cells
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue