Korean Diabetes J.
2010 Aug;34(4):211-219. 10.4093/kdj.2010.34.4.211.
O-GlcNAc Modification: Friend or Foe in Diabetic Cardiovascular Disease
- Affiliations
-
- 1Department of Medical Sciences, Kyungpook National University School of Medicine, Daegu, Korea.
- 2Department of Fundamental Medical and Pharmaceutical Sciences, Catholic University of Daegu CU Leaders' College, Gyeongsan, Korea. syjeoung@cu.ac.kr
- KMID: 2029873
- DOI: http://doi.org/10.4093/kdj.2010.34.4.211
Abstract
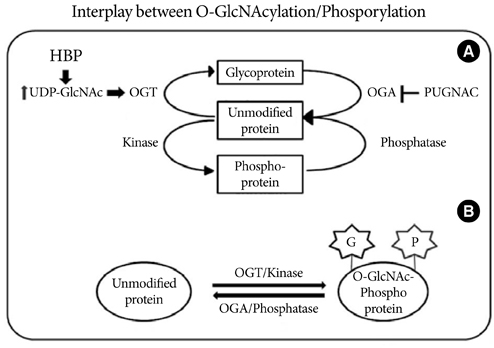
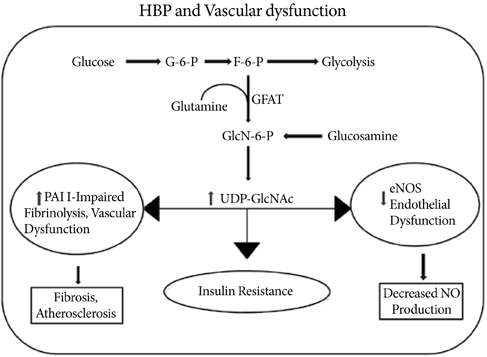
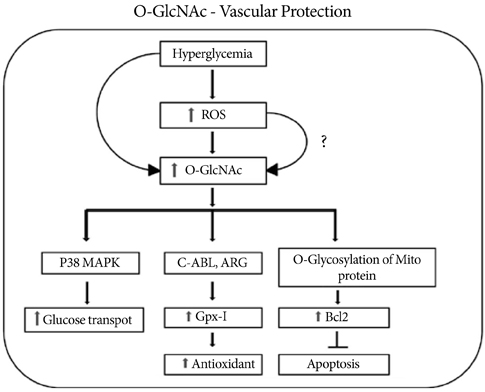
- O-Linked beta-N-acetyl glucosaminylation (O-GlcNAcylation) is a dynamic post-translational modification that occurs on serine and threonine residues of cytosolic and nuclear proteins in all cell types, including those involved in the cardiovascular system. O-GlcNAcylation is thought to act in a manner analogous to protein phosphorylation. O-GlcNAcylation rapidly cycles on/off proteins in a time scale similar to that for phosphorylation/dephosphorylation of proteins. Several studies indicate that O-GlcNAc might induce nuclear localization of some transcription factors and may affect their DNA binding activities. However, at the cellular level, it has been shown that O-GlcNAc levels increase in response to stress and augmentation of this response suppresses cell survival. Increased levels of O-GlcNAc have been implicated as a pathogenic contributor to glucose toxicity and insulin resistance, which are major hallmarks of type 2 diabetes and diabetes-related cardiovascular complications. Thus, O-GlcNAc and its metabolic functions are not yet well-understood; focusing on the role of O-GlcNAc in the cardiovascular system is a viable target for biomedical investigation. In this review, we summarize our current understanding of the role of O-GlcNAc on the regulation of cell function and survival in the cardiovascular system.
MeSH Terms
-
Acetylglucosaminidase
Cardiovascular Diseases
Cardiovascular System
Cell Survival
Cytosol
Diabetes Mellitus, Type 2
DNA
Friends
Glucose
Humans
Insulin Resistance
Nuclear Proteins
Phosphorylation
Protein Processing, Post-Translational
Proteins
Serine
Threonine
Transcription Factors
Vascular Diseases
Acetylglucosaminidase
DNA
Glucose
Nuclear Proteins
Proteins
Serine
Threonine
Transcription Factors
Figure
Reference
-
1. Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008. 295:E17–E28.2. Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001. 291:2376–2378.3. Guinez C, Morelle W, Michalski JC, Lefebvre T. O-GlcNAc glycosylation: a signal for the nuclear transport of cytosolic proteins? Int J Biochem Cell Biol. 2005. 37:765–774.4. Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004. 1673:13–28.5. Butkinaree C, Park K, Hart GW. O-linked β-N-acetyl glucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010. 1800:96–106.6. Bouche C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type-2 diabetes. Endocr Rev. 2004. 25807–25830.7. Hanover JA. Glycan-dependent signaling: O-linked N-acetyl glucosamine. FASEB J. 2001. 15:1865–1876.8. Buse MG. Hexosamines, insulin resistance, and the complications of diabetes; current status. Am J Physiol Endocrinol Metab. 2006. 290:E1–E8.9. Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967. 242:3135–3141.10. Marshall S, Nadeau O, Yamasaki K. Dynamic actions of glucose and glucosamine on Hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetyl glucosamine and ATP levels. J Biol Chem. 2004. 279:35313–35319.11. Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytosolic proteins. Identification of a uridine diphospho-N-acetyl glucosamine: peptide beta-Nacetylglucosaminyl transferase. J Biol Chem. 1990. 265:2563–2568.12. Shafi R, Lyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The O-GlcNAc transferase gene resides on the X-chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000. 97:5735–5739.13. Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci U S A. 2008. 105:13793–13798.14. Defronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes care. 1991. 14:173–194.15. Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of Hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991. 266:4706–4712.16. Marshall S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional prospective of diabetes, obesity and cancer. Sci STKE. 2006. 2006:re7.17. Rajapakse AG, Ming XF, Carvas JM, Yang Z. The hexosamine biosynthesis inhibitor azaserine prevents endothelial inflammation and dysfunction under hyperglycemic condition through antioxidant effects. Am J Physiol Heart Circ Physiol. 2009. 296:H815–H822.18. Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, Shi Y. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006. 55:93–101.19. Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in AKT activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002. 99:5313–5318.20. Cooksey RC, Hebert LF Jr, Zhu JH, Wofford P, Garrey WT, McClain DA. Mechanism of Hexosamine-induced insulin resistance in transgenic mice over-expressing glutamine: fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology. 1999. 140:1151–1157.21. Brozinick JT Jr, Berkemeier BA, Elmendorf JS. Acting on GLUT4: membrane and cytoskeletal components of insulin action. Curr Diabetes Rev. 2007. 3:111–122.22. Bhonagiri P, Pattar GR, Horvath EM, Habegger KM, McCarthy AM, Elmendof JS. Hexosamine biosynthesis pathway flux contributes to insulin resistance via altering membrane phosphatidylinositol 4, 5-bisphosphate and cortical filamentous actin. Endocrinology. 2009. 150:1636–1645.23. Gandy JC, Rountree AE, Bijur GN. Akt is dynamically modified with O-GlcNAc following treatments with PUGNAC and insulin-like growth factor-1. FEBS Lett. 2006. 580:3051–3058.24. Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signaling links O-GlcNAc transferase to insulin resistance. Nature. 2008. 451:964–969.25. Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005. 109:143–159.26. Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szado C, Brownlee M. Inhibition of GAPDH activity by poly (ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003. 112:1049–1057.27. Nerlich AG, Sauer U, Kolm-Lilly V, Wagner E, Koch M, Schleicher ED. Expression of glutamine: fructose 6-phosphate amidotransferase in human tissues; evidence for high variability and distinct regulation in diabetes. Diabetes. 1998. 47:170–178.28. Hall JL, Chatham JC, Eldar-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis: role of GSK3beta. Diabetes. 2001. 50:1171–1179.29. Akimoto Y, Kreppel LK, Hirano H, Hart GW. Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells; elevated expression and altered patterns of O-GlcNAcylation. Arch Biochem Biophys. 2001. 389:166–175.30. McClain DA, Paterson AJ, Roos MD, Wei X, Kudlow JE. Glucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1992. 89:8150–8154.31. Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetyl glucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005. 280:32944–32956.32. Williams B, Schrier RW. Characterization of glucose-induced in situ protein kinase C activity in cultured vascular smooth muscle cells. Diabetes. 1992. 41:1464–1472.33. Hattori Y, Hattori S, Sato N, Kasai K. High-glucose-induced nuclear factor kB activation in vascular smooth muscle cells. Cardiovasc Res. 2000. 46:188–197.34. Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NF-kB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008. 105:17345–17350.35. Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007. 73:288–297.36. Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010. 55:180–188.37. Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med. 2005. 230:829–835.38. Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003. 278:44230–44237.39. Song M, Kim HS, Park JM, Kim SH, Kim IH, Ryu SH, Suh PG. O-GlcNAc transferase is activated by CAMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal. 2008. 20:94–104.40. Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007. 104:109–115.41. Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked Beta-N-acetyl glucosaminyl transferease substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008. 283:33935–33941.42. Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001. 22:36–52.43. Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, Yki-Jarvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin dependent diabetes mellitus. Circulation. 1996. 94:1276–1282.44. Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006. 45:268–276.45. Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by post-translational modification at the AKT site. J Clin Invest. 2001. 108:1341–1348.46. Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002. 106:466–472.47. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000. 97:12222–12226.48. Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in micro vascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007. 282:31038–31045.49. Hata Y, Duh E, Zhang K, Robinson GS, Aiello LP. Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J Biol Chem. 1998. 273:19294–19303.50. Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation. 1997. 96:1152–1156.51. Sohn KC, Lee KY, Park JE, Do SI. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem Biophys Res Commun. 2004. 322:1045–1051.52. Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004. 279:30133–30142.53. Lim KH, Chang HI. O-linked N-acetyl glucosamine suppresses thermal aggregation of Sp1. FEBS Lett. 2006. 580:4645–4652.54. Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluinal arterial injury. Am J Physiol Heart Circ Physiol. 2008. 295:H335–H342.55. Zou L, Yang S, Champattanachai V, Shunhua HU, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-kB signaling. Am J Physiol Heart Circ Physiol. 2009. 296:H515–H523.56. Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007. 292:C178–C187.57. Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000. 278:H1948–H1954.58. Ngoh GA, Jones SP. O-GlcNAc signaling attenuates mitochondrial membrane permeability transition. FASEB J. 2008. 22:1130.8. [Meeting Abstract].59. Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008. 294:C1509–C1520.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Shank3 a ‘Friend or Foe’ of the Heart? Its Role in Cardiac Calcium Homeostasis
- O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes
- Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy
- Anti-tumor Necrosis Factor Therapy for Crohn Disease: Friend or Foe to the Surgeon?
- Glucose in Perinatal Hypoxic-Ischemic Brain Injury: Friend or Foe?