J Clin Neurol.
2006 Dec;2(4):252-257. 10.3988/jcn.2006.2.4.252.
1alpha,25-Dihydroxyvitamin D3 Protects Dopaminergic Neurons in Rodent Models of Parkinson's Disease through Inhibition of Microglial Activation
- Affiliations
-
- 1Department of Neurology, The Catholic University of Korea, Seoul, Korea. nuyikim@catholic.ac.kr
- KMID: 1980465
- DOI: http://doi.org/10.3988/jcn.2006.2.4.252
Abstract
- BACKGROUND
Recent studies have demonstrated the molecular basis of the immunomodulatory and anti-inflammatory activities of 1,25-dihydroxyvitamin D3(1,25-(OH)2D3). This hormone improves behavioral deficits and normalizes the nigral dopamine levels in animal models of Parkinson's disease (PD).
METHODS
We studied whether the administration of 1,25-(OH)2D3 would protect against 6-hydroxydopa (6-OHDA)- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neuronal injury, and its potential regulatory effect on microglia activation.
RESULTS
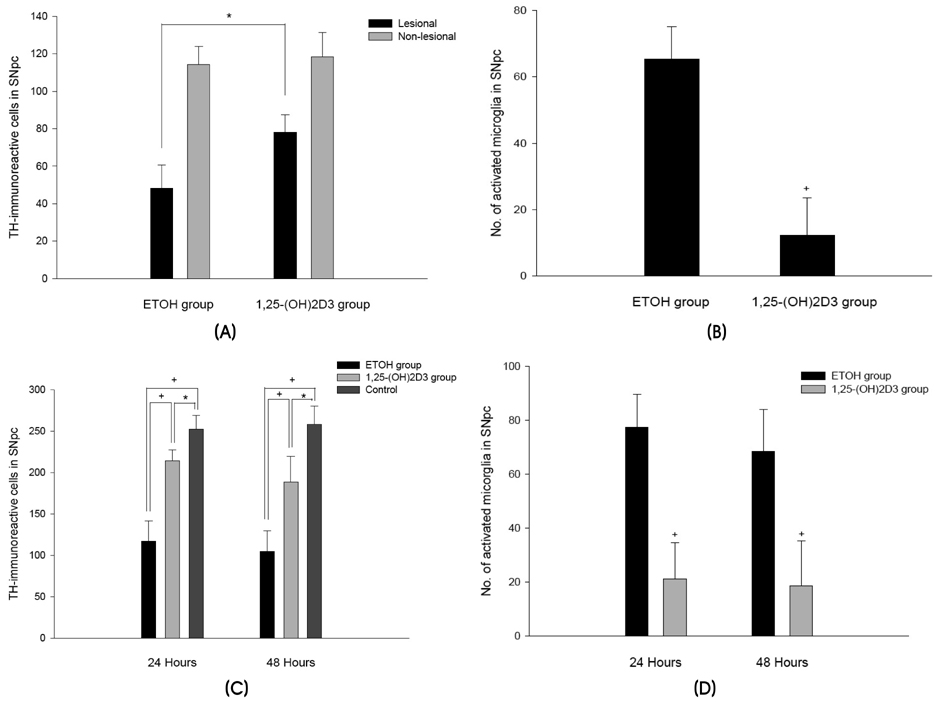
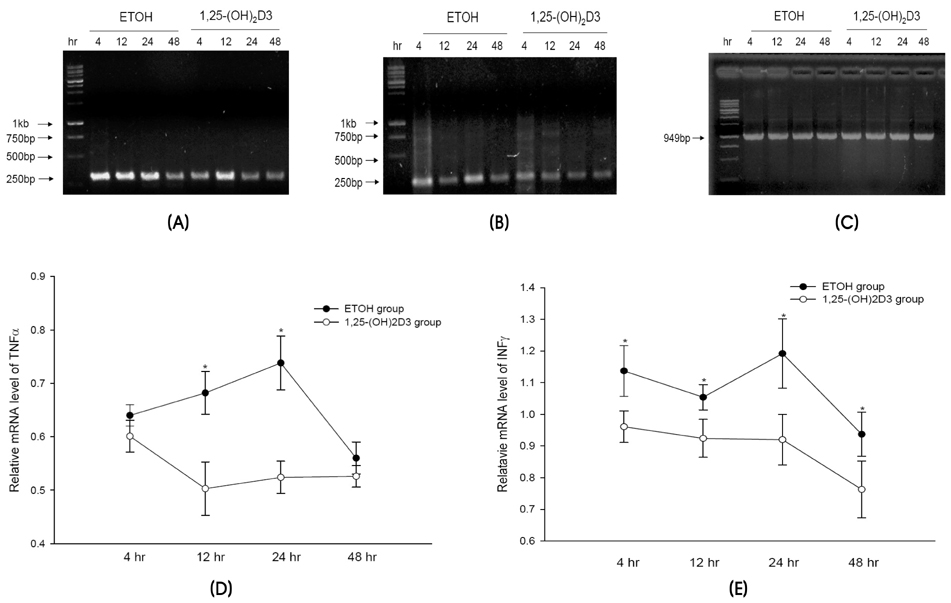
We found that 1,25-(OH)2D3 pretreatment significantly decreased 6-OHDA- and MPTP-induced dopaminergic neuronal loss in the substantia nigra pars compacta by preventing the activation of microglia. This observed neuroprotective effect in MPTP-treated mice that were given 1,25-(OH)2D3 may be attributable to inhibition of proinflammatory cytokine expression.
CONCLUSION
These results suggest that 1,25-(OH)2D3 is a potentially valuable neuroprotective agent; it may therefore be considered for the treatment of pathologic conditions of the central nervous system, such as PD, where inflammation-induced neurodegeneration occurs.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Animals
Central Nervous System
Dopamine
Dopaminergic Neurons*
Inflammation
Mice
Microglia
Models, Animal
Neurons
Neuroprotective Agents
Parkinson Disease*
Rats
Rodentia*
Substantia Nigra
Vitamin D
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Dopamine
Neuroprotective Agents
Vitamin D
Figure
Reference
-
1. Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003. 304:1–7.
Article2. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988. 38:1285–1291.
Article3. May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004. 3:377–393.4. Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxy-vitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996. 7:2171–2175.
Article5. Sanchez B, Lopez-Martin E, Segura C, Labandeira-Garcia JL, Perez-Fernandez R. 1,25-Dihydroxyvitamin D(3) increases striatal GDNF mRNA and protein expression in adult rats. Brain Res Mol Brain Res. 2002. 108:143–146.
Article6. Ryu SY, Kim JS, Choi YB, Han SR, Park JW, Park SK, et al. The Effect of 1,25-Dihydroxyvitamin D3 on dopaminergic neurons and microglial activation in Parkinsonian rat model induced by 6-hydroxydopamine. J Korean Neurol Assoc. 2005. 23:368–373.7. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 1986. 2nd ed. Sydney, Australia: Academic Press.8. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 1997. 1st ed. San Diego: Academic Press.9. Ciesielska A, Joniec I, Przybylkowski A, Gromadzka G, Kurkowska-Jastrzebska I, Czlonkowska A, et al. Dynamics of expression of the mRNA for cytokines and inducible nitric synthase in a murine model of the Parkinson's disease. Acta Neurobiol Exp (Wars). 2003. 63:117–126.10. Gerlach M, Riederer P. Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm. 1996. 103:987–1041.
Article11. Kurosaki R, Muramatsu Y, Kato H, Araki T. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2004. 78:143–153.
Article12. Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des. 2005. 11:999–1016.
Article13. Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996. 380:252–255.
Article14. Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res. 2000. 62:374–382.
Article15. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998. 22:282–294.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of 1, 25-Dihydroxyvitamin D3 on Dopaminergic Neurons and Microglial Activation in Parkinsonian Rat Model Induced by 6-Hydroxydopamine
- Neuroprotection by 1,25(OH)2-Vitamin D3 Against Kainic Acid-Induced Excitotoxicity in the Rat Hippocampus
- Prothrombin Kringle-2: A Potential Inflammatory Pathogen in the Parkinsonian Dopaminergic System
- Morphological Changes in Dopaminergic Neurons in Selective Parkinson's Rat Model
- L-PDMP, Ganglioside Synthesis Enhancer Protects Dopaminergic Neurons from Death by Proteasomal Inhibition and the Accumulation of alpha-synuclein