Int J Stem Cells.
2014 Nov;7(2):153-157. 10.15283/ijsc.2014.7.2.153.
Exogenous c-Myc Blocks Differentiation and Improves Expansion of Human Erythroblasts In vitro
- Affiliations
-
- 1Department of Basic Science Research, Cellologi, LLC, USA. contact@cellologi.com
- 2Santa Barbara Cottage Hospital, Santa Barbara, USA.
- KMID: 1974603
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.153
Abstract
- BACKGROUND
Engineered blood has the greatest potential to combat a predicted future shortfall in the blood supply for transfusion treatment. The production of red blood cells from hematopoietic stem cells in the laboratory is possible but the mass production of red blood cells to the level present in a blood transfusion unit is currently not possible. The proliferation capacity of the immature red blood cell will need to be increased to enable mass production. This work focused on the hypothesis that exogenous c-Myc can delay the differentiation process of highly proliferative immature erythroblasts, and increase the proliferation capacity of erythroblast cell cultures.
OBJECTIVES
The objective of this research effort was to improve in vitro erythropoiesis from stem cells without gene transfection with the eventual goal of producing blood for transfusion treatment in a manner that could be easily translated into clinical medicine.
METHODS
The hematopoietic stem cell containing mononuclear cell fraction of venous blood samples was cultured in a liquid media containing erythroblasts growth factors with and without exogenous c-Myc combined with a cell-penetrating peptide. The cells were maintained in the liquid culture media for 23 days. Viable cells were counted and analyzed with flow cytometry.
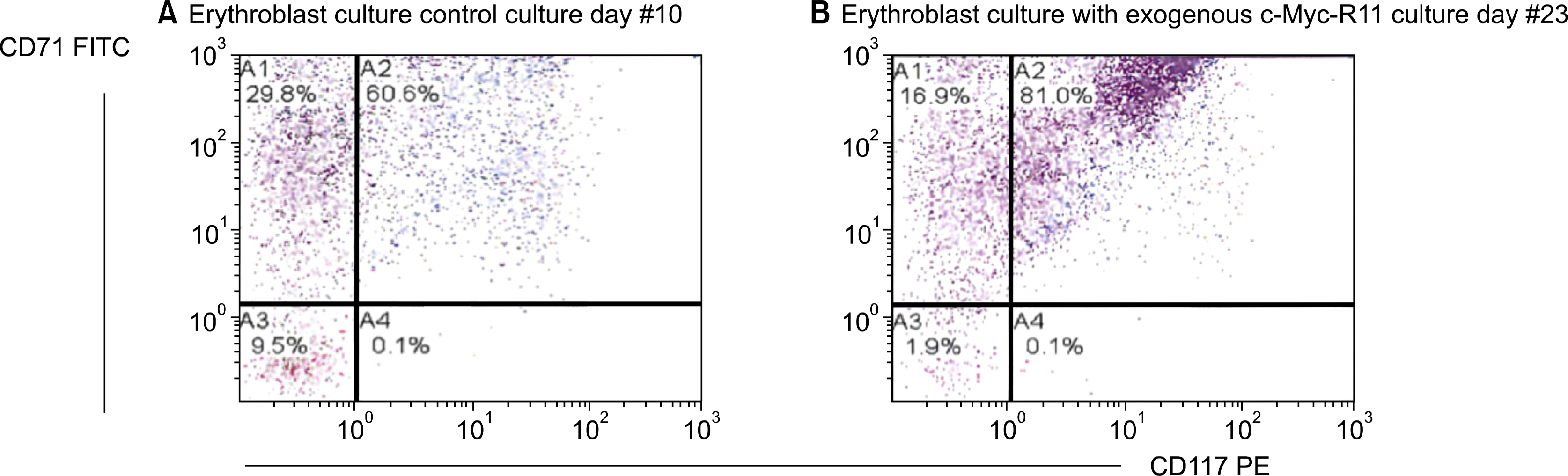
RESULTS
Our results show a 4 fold increase in expansion of the erythroblasts grown in the c-Myc containing growth media compared to the control. Eighty percent of these cells retained the CD117 surface receptor, indicating immature cells.
CONCLUSION
Exogenous c-Myc blocks the differentiation and improves in vitro expansion of human erythroblasts.
Keyword
MeSH Terms
-
Adult Stem Cells
Blood Transfusion
Cell Culture Techniques
Clinical Medicine
Culture Media
Erythroblasts*
Erythrocytes
Erythropoiesis
Flow Cytometry
Hematopoietic Stem Cells
Humans
Intercellular Signaling Peptides and Proteins
Proto-Oncogene Proteins c-myc
Stem Cells
Transfection
Culture Media
Intercellular Signaling Peptides and Proteins
Proto-Oncogene Proteins c-myc
Figure
Cited by 1 articles
-
Genetically Engineered In Vitro Erythropoiesis
Cristopher Geiler, Inez Andrade, Alexandra Clayton, Daniel Greenwald
Int J Stem Cells. 2016;9(1):53-59. doi: 10.15283/ijsc.2016.9.1.53.
Reference
-
References
1. The 2007 National Blood Collection and Utilization Survey Report. Washington DC: DHHS;2008.2. Benjamin RJ, Whitaker BI. Boom or bust? Estimating blood demand and supply as the baby boomers age. Transfusion. 2011. 51:670–673.
Article3. Surgenor DM, Wallace EL, Hao SH, Chapman RH. Collection and transfusion of blood in the United States, 1982–1988. N Engl J Med. 1990. 322:1646–1651.
Article4. Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993. 33:139–144.
Article5. Wallace EL, Churchill WH, Surgenor DM, An J, Cho G, McGurk S, Murphy L. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995. 35:802–812.
Article6. Sullivan MT, McCullough J, Schreiber GB, Wallace EL. Blood collection and transfusion in the United States in 1997. Transfusion. 2002. 42:1253–1260.
Article7. Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005. 45:141–148.
Article8. Blood Facts and Statistics. American Red Cross. www.redcrossblood.org/learn-about-blood/blood-facts-and-statistics.9. Stephenson JR, Axelrad AA, McLeod DL, Shreeve MM. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971. 68:1542–1546.
Article10. Nathan DG, Chess L, Hillman DG, Clarke B, Breard J, Merler E, Housman DE. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978. 147:324–339.
Article11. Brendt P, Rehfeld I, Kamphausen A, Kreissig C, Peters J. Lipopolysaccharide interference in erythropoiesis in mice. Anaesthesia. 2012. 67:493–500.
Article12. Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005. 15:146–155.
Article13. Lodish H, Flygare J, Chou S. From stem cell to erythroblast: regulation of red cell production at multiple levels by multiple hormones. IUBMB Life. 2010. 62:492–496.
Article14. Spangler R, Sytkowski AJ. c-myc is an erythropoietin early response gene in normal erythroid cells: evidence for a protein kinase C-mediated signal. Blood. 1992. 79:52–57.
Article15. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002. 2:489–501.
Article16. Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000. 16:653–699.
Article17. Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002. 2:764–776.
Article18. Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006. 25:2723–2734.
Article19. Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006. 16:253–264.
Article20. Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008. 27:6462–6472.
Article21. Hoffman B, Amanullah A, Shafarenko M, Liebermann DA. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene. 2002. 21:3414–3421.
Article22. Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004. 18:2747–2763.
Article23. Acosta JC, Ferrándiz N, Bretones G, Torrano V, Blanco R, Richard C, O'Connell B, Sedivy J, Delgado MD, León J. Myc inhibits p27-induced erythroid differentiation of leukemia cells by repressing erythroid master genes without reversing p27-mediated cell cycle arrest. Mol Cell Biol. 2008. 28:7286–7295.
Article24. Data Generated in-house STEMCELL Technologies Inc. 570 West Seventh Avenue, Suite 400, Vancouver, BC, V5Z 1B3, Canada.25. Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004. 103:4310–4316.
Article26. Dorn I, Lazar-Karsten P, Boie S, Ribbat J, Hartwig D, Driller B, Kirchner H, Schlenke P. In vitro proliferation and differentiation of human CD34+ cells from peripheral blood into mature red blood cells with two different cell culture systems. Transfusion. 2008. 48:1122–1132.
Article27. Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001. 414:768–773.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maturation of Erythroblasts in Human Liver during Ontogeny - An Electron Microscopic Study
- Comparison of Serum-Free Media in RBC Differentiation from Human Hematopoietic Stem Cells
- Genetically Engineered In Vitro Erythropoiesis
- c-Myc expression is related with cell proliferation and associated with poor clinical outcome in human gastric cancer
- Increased expression of the c-fos and c-myc oncogenes in psoriatic lesions