Diabetes Metab J.
2011 Oct;35(5):469-479. 10.4093/dmj.2011.35.5.469.
Protective Effect of Heme Oxygenase-1 on High Glucose-Induced Pancreatic beta-Cell Injury
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. ybahn@catholic.ac.kr
- KMID: 1857502
- DOI: http://doi.org/10.4093/dmj.2011.35.5.469
Abstract
- BACKGROUND
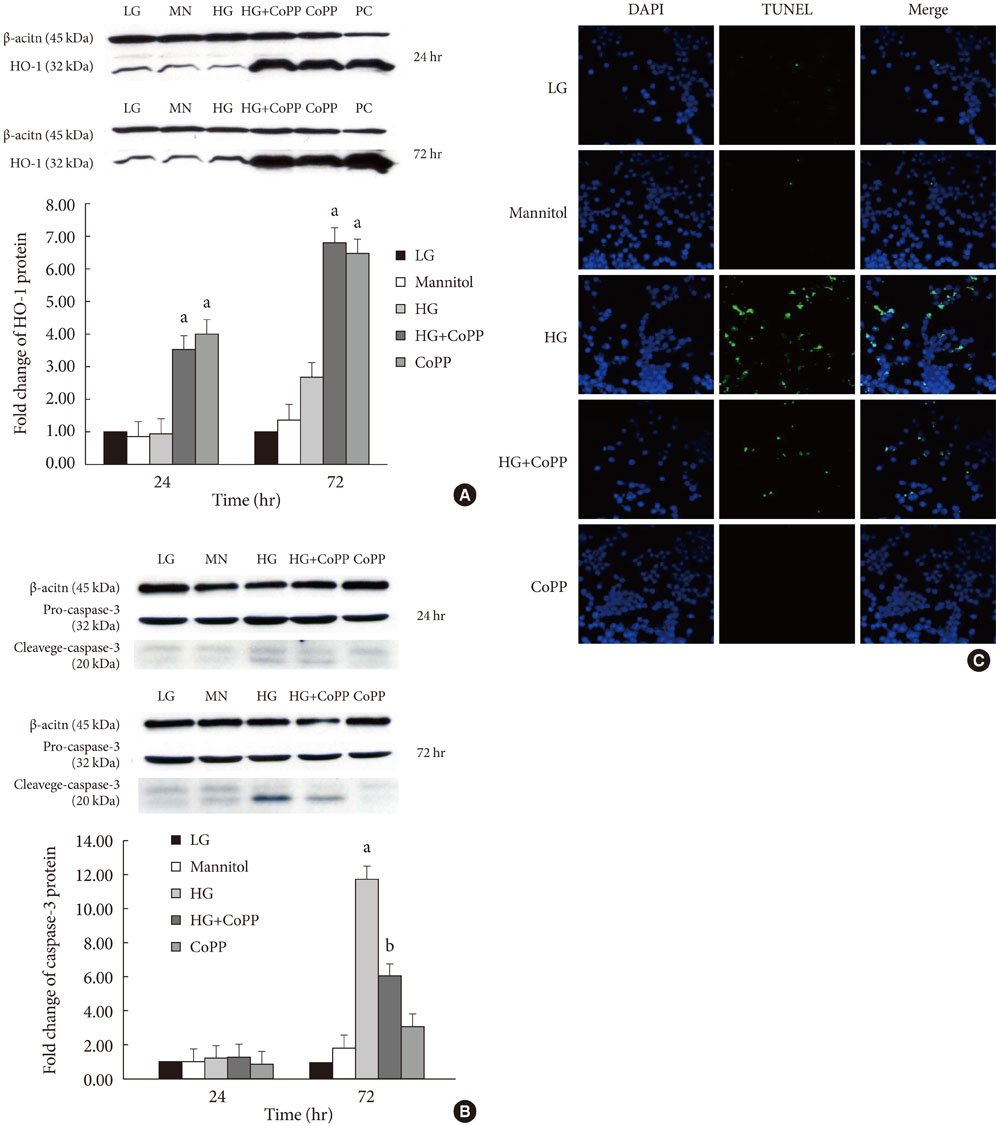
Glucose toxicity that is caused by chronic exposure to a high glucose concentration leads to islet dysfunction and induces apoptosis in pancreatic beta-cells. Heme oxygenase-1 (HO-1) has been identified as an anti-apoptotic and cytoprotective gene. The purpose of this study is to investigate whether HO-1 up-regulation when using metalloprotophyrin (cobalt protoporphyrin, CoPP) could protect pancreatic beta-cells from high glucose-induced apoptosis.
METHODS
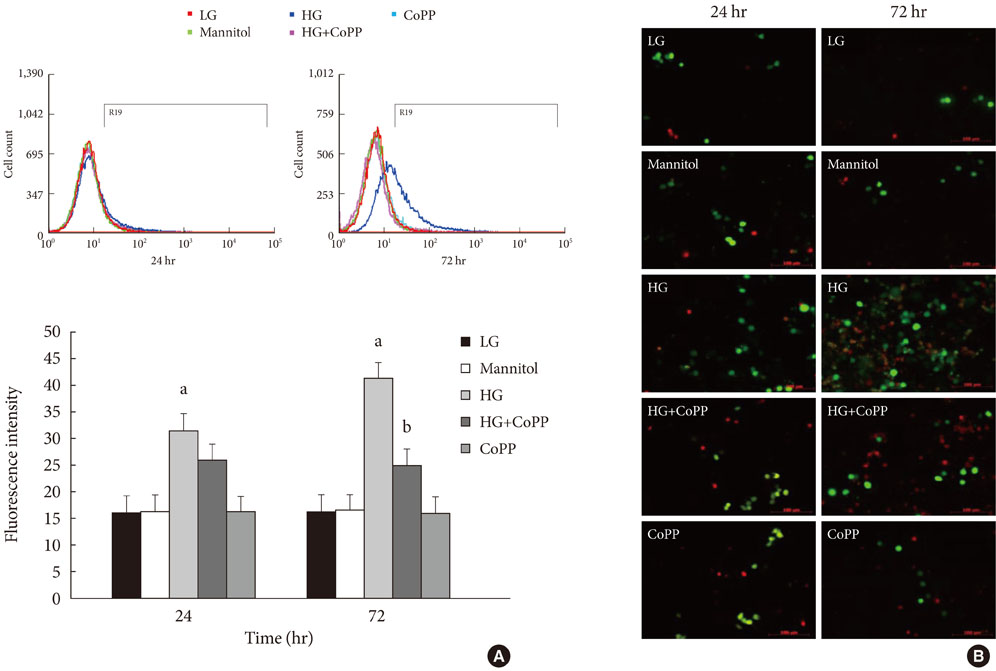
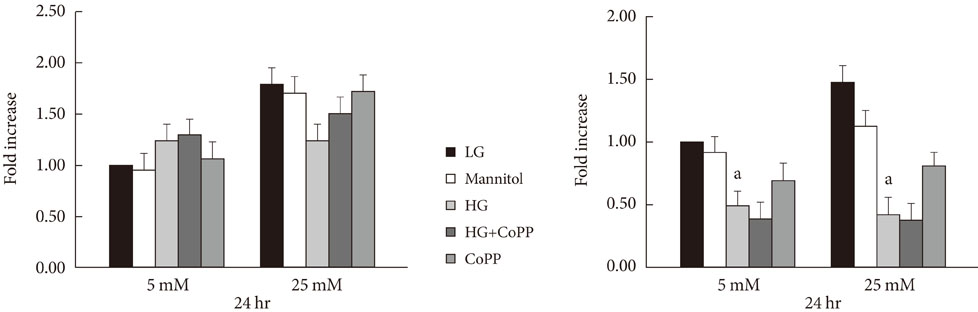
Reverse transcription-polymerase chain reaction was performed to analyze the CoPP-induced mRNA expression of HO-1. Cell viability of INS-1 cells cultured in the presence of CoPP was examined by acridine orange/propidium iodide staining. The generation of intracellular reactive oxygen species (ROS) was measured using flow cytometry. Glucose stimulated insulin secretion (GSIS) was determined following incubation with CoPP in different glucose concentrations.
RESULTS
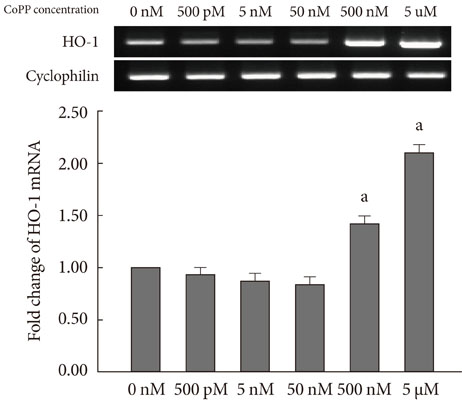
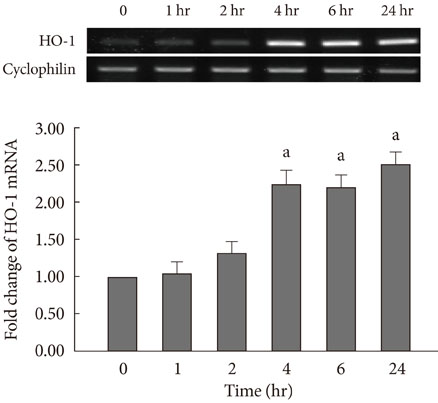
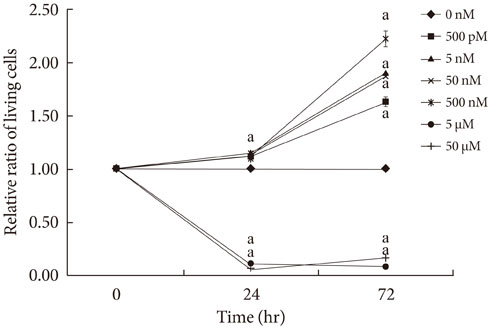
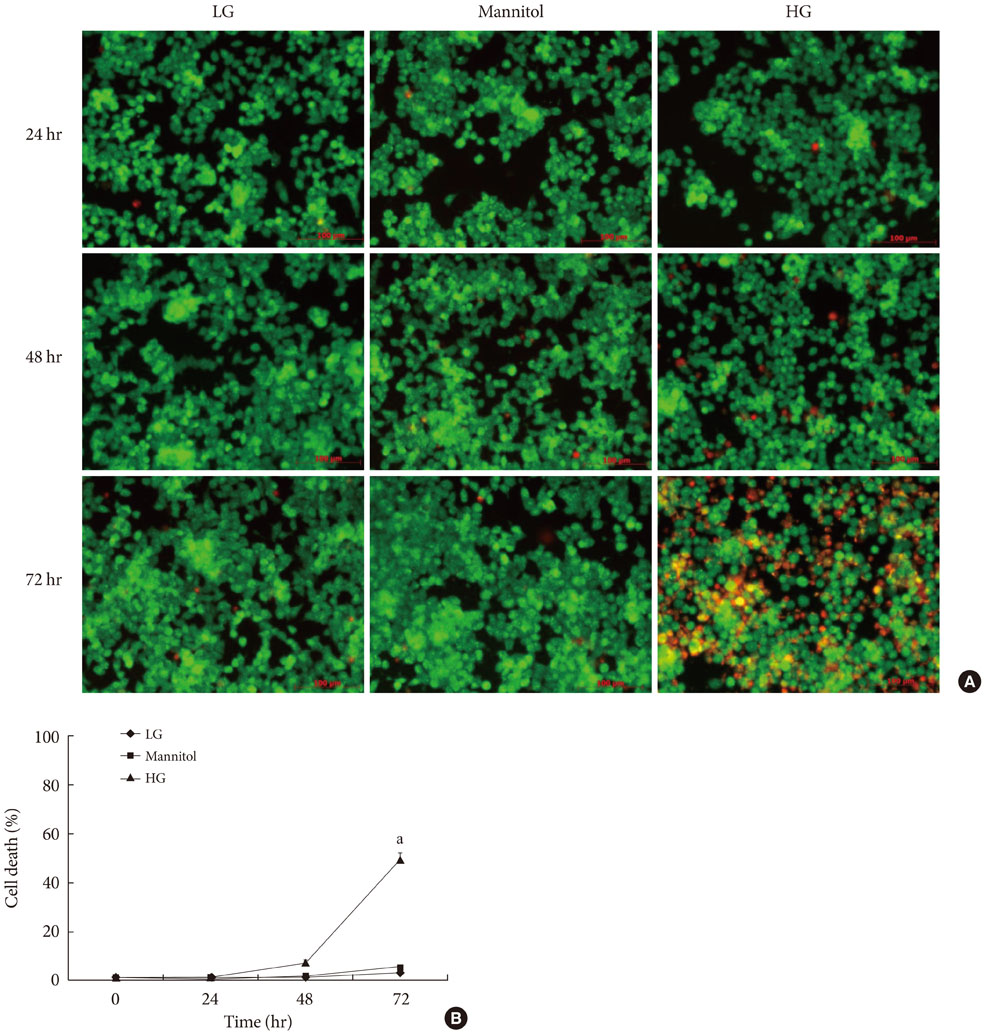
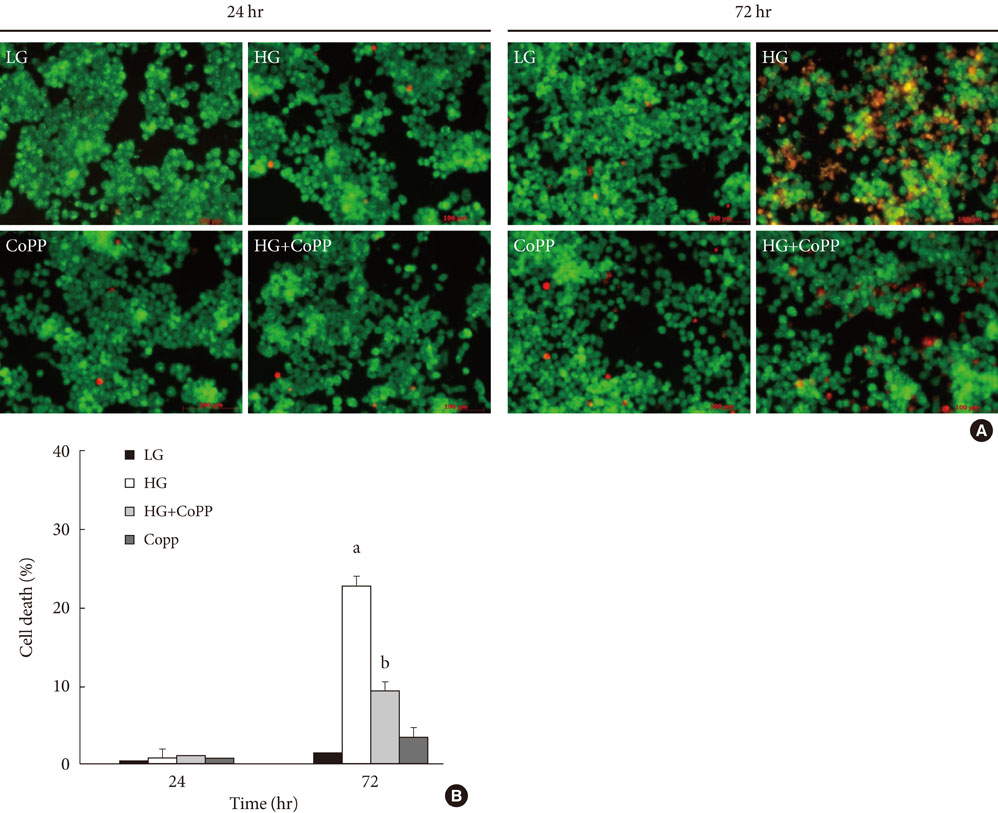
CoPP increased HO-1 mRNA expression in both a dose- and time-dependent manner. Overexpression of HO-1 inhibited caspase-3, and the number of dead cells in the presence of CoPP was significantly decreased when exposed to high glucose conditions (HG). CoPP also decreased the generation of intracellular ROS by 50% during 72 hours of culture with HG. However, decreased GSIS was not recovered even in the presence of CoPP.
CONCLUSION
Our data suggest that CoPP-induced HO-1 up-regulation results in protection from high glucose-induced apoptosis in INS-1 cells; however, glucose stimulated insulin secretion is not restored.
MeSH Terms
Figure
Cited by 1 articles
-
Diabetes and Alzheimer's Disease: Mechanisms and Nutritional Aspects
Hee Jae Lee, Hye In Seo, Hee Yun Cha, Yun Jung Yang, Soo Hyun Kwon, Soo Jin Yang
Clin Nutr Res. 2018;7(4):229-240. doi: 10.7762/cnr.2018.7.4.229.
Reference
-
1. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999. 104:787–794.2. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003. 46:3–19.3. Hou ZQ, Li HL, Gao L, Pan L, Zhao JJ, Li GW. Involvement of chronic stresses in rat islet and INS-1 cell glucotoxicity induced by intermittent high glucose. Mol Cell Endocrinol. 2008. 291:71–78.4. Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002. 99:12363–12368.5. Sumoski W, Baquerizo H, Rabinovitch A. Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia. 1989. 32:792–796.6. Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008. 57:1952–1965.7. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997. 46:1733–1742.8. Tiedge M, Lortz S, Munday R, Lenzen S. Protection against the co-operative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia. 1999. 42:849–855.9. Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1beta-induced cytotoxicity and reduces nitric oxide production. J Clin Invest. 1998. 101:1811–1820.10. Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY. Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting cells. Diabetologia. 2000. 43:625–631.11. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.12. Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-alpha and interleukin-1 alpha on heme oxygenase-1 expression in human endothelial cells. Am J Physiol. 1998. 274(3 Pt 2):H883–H891.13. Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997. 80:557–564.14. Ye J, Laychock SG. A protective role for heme oxygenase expression in pancreatic islets exposed to interleukin-1beta. Endocrinology. 1998. 139:4155–4163.15. Piro S, Anello M, Di Pietro C, Lizzio MN, Patane G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002. 51:1340–1347.16. Mellado-Gil JM, Aguilar-Diosdado M. High glucose potentiates cytokine- and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genes. J Endocrinol. 2004. 183:155–162.17. Halliwell B, Gutteridge JM. Free radical in biology and medicine. 1989. 2nd ed. Oxford: Clarendon Press.18. Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C. A20 inhibits cytokine-induced apoptosis and nuclear factor kappaB-dependent gene activation in islets. J Exp Med. 1999. 190:1135–1146.19. Won KC, Moon JS, Eun MJ, Yoon JS, Chun KA, Cho IH, Kim YW, Lee HW. A protective role for heme oxygenase-1 in INS-1 cells and rat islets that are exposed to high glucose conditions. J Korean Med Sci. 2006. 21:418–424.20. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988. 2:2557–2568.21. Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001. 50:1983–1991.22. Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999. 1:152–157.23. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999. 48:927–932.24. Ahn YB, Xu G, Marselli L, Toschi E, Sharma A, Bonner-Weir S, Sgroi DC, Weir GC. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia. 2007. 50:334–342.25. Tajiri Y, Moller C, Grill V. Long-term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology. 1997. 138:273–280.26. Chen H, Li X, Epstein PN. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005. 54:1437–1446.27. Woo J, Iyer S, Mori N, Buelow R. Alleviation of graft-versus-host disease after conditioning with cobalt-protoporphyrin, an inducer of heme oxygenase-1. Transplantation. 2000. 69:623–633.28. Chen X, Zhang Z, Su C, Gu W, Li H, Zhou G. Protective effect of heme oxygenase-1 to pancreas islet xenograft. J Surg Res. 2010. 164:336–343.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Protective Role for Heme Oxygenase-1 in INS-1 Cells and Rat Islets that are Exposed to High Glucose Conditions
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Change of Expression and Activity of Heme Oxygenase-1 in Rat Corpus Cavernosum during Low-flow Priapism
- Nrf2-Heme oxygenase-1 modulates autophagy and inhibits apoptosis triggered by elevated glucose levels in renal tubule cells
- Heme Oxygenase-1 Induced by Aprotinin Inhibits Vascular Smooth Muscle Cell Proliferation Through Cell Cycle Arrest in Hypertensive Rats