J Korean Med Sci.
2006 Jun;21(3):418-424. 10.3346/jkms.2006.21.3.418.
A Protective Role for Heme Oxygenase-1 in INS-1 Cells and Rat Islets that are Exposed to High Glucose Conditions
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Yeungnam University, Daegu, Korea. kcwon@med.yu.ac.kr
- 2Department of Nuclear Medicine, College of Medicine, Yeungnam University, Daegu, Korea.
- 3Department of Physiology, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 1778421
- DOI: http://doi.org/10.3346/jkms.2006.21.3.418
Abstract
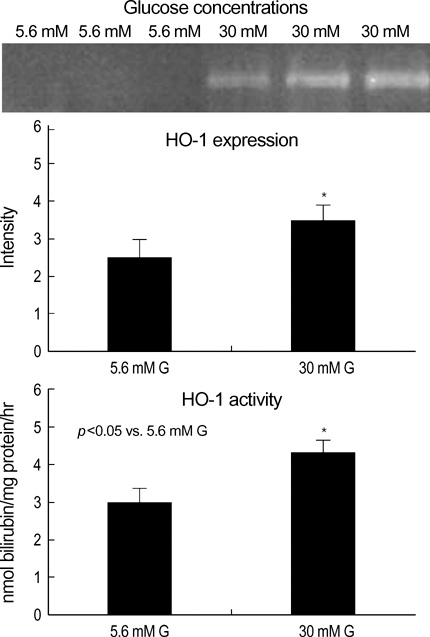
- Heme oxygenase-1 (HO-1) has been described as an inducible protein that is capable of cytoprotection via radical scavenging and the prevention of apoptosis. Chronic exposure to hyperglycemia can lead to cellular dysfunction that may become irreversible over time, and this process has been termed glucose toxicity. Yet little is known about the relation between glucose toxicity and HO-1 in the islets. The purposes of the present study were to determine whether prolonged exposure of pancreatic islets to a supraphysiologic glucose concentration disrupts the intracellular balance between reactive oxygen species (ROS) and HO-1, and so this causes defective insulin secretion; we also wanted to evaluate a protective role for HO-1 in pancreatic islets against high glucose levels. The intracellular peroxide levels of the pancreatic islets (INS-1 cell, rat islet) were increased in the high glucose media (30 mM glucose or 50 mM ribose). The HO-1 expression was induced in the INS-1 cells by the high glucose levels. Both the HO-1 expression and glucose stimulated insulin secretion (GSIS) was decreased simultaneously in the islets by treatment of the HO-1 antisense. The HO-1 was upregulated in the INS-1 cells by hemin, an inducer of HO-1. And, HO-1 upregulation induced by hemin reversed the GSIS in the islets at a high glucose condition. These results suggest HO-1 seems to mediate the protective response of pancreatic islets against the oxidative stress that is due to high glucose conditions.
MeSH Terms
Figure
Reference
-
1. Ye J, Laychock SG. A protective role for heme oxygenase expression in pancreatic islets exposed to interleukin-1β. Endocrinology. 1998. 139:4155–4163.2. Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999. 291:416–423.3. Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001. 50:1983–1991.
Article4. Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentration. J Clin Invest. 1992. 90:320–325.5. Won KC. Oxidative stress in pancreatic islet beta-cells exposed to high glucose concentration. J Korean Diabetes Assoc. 2004. 28:250–254.6. Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005. 280:11107–11113.
Article7. Sharma A, Olson LK, Robertson RP, Stein R. The reduction of insulin gene transcription in HIT-T15 beta cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcription factor expression. Mol Endocrinol. 1995. 9:1127–1134.
Article8. Harmon JS, Tanaka Y, Olson LK, Robertson RP. Reconstitution of glucotoxic HIT-T15 cells with somatostatin transcription factor-1 partially restores insulin promoter activity. Diabetes. 1998. 47:900–904.
Article9. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.
Article10. Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes. 1998. 47:1578–1585.
Article11. Kralik PM, Xu B, Epstein PN. Catalase transfection decreases hydrogen peroxide toxicity in a pancreatic beta cell line. Endocr Res. 1998. 24:79–87.12. Tiedge M, Lortz S, Munday R, Lenzen S. Protection against the cooperative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia. 1999. 42:849–855.
Article13. Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1β-induced cytotoxicity and reduces nitric oxide production. J Clin Invest. 1998. 101:1811–1820.14. Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY. Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity n human islets and INS-1 insulin-secreting cells. Diabetologia. 2000. 43:625–631.15. Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992. 130:167–178.
Article16. Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002. 99:12363–12368.17. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem J. 1987. 245:243–250.
Article18. Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.
Article19. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981. 199:393–398.
Article20. Oliveira HR, Curi R, Carpinelli AR. Glucose induces an acute increase of superoxide dismutase activity in incubated rat pancreatic islets. Am J Physiol Cell Physiol. 1999. 276:507–510.21. McDonagh AF. Is bilirubin good for you? Clin Perinatol. 1990. 17:359–369.
Article22. George JW, Nulk K, Weiss A, Bruss ML, Cornelius CE. Biliverdin reductase activity in cattle, sheep, rabbits and rats. Int J Biochem. 1989. 21:477–481.
Article23. Helqvist S, Polla BS, Johannesen J, Nerup J. Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1β. Diabetologia. 1991. 34:150–156.
Article24. Henningsson R, Alm P, Lundquist I. Occurrence and putative hormone regulatory function of a constitutive heme oxygenase in rat pancreatic islets. Am J Physiol Cell Physiol. 1997. 273:703–709.
Article25. Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodstrom M, Mello M, Andersson A, Pipeleers DG, Hellerstrom C, Eizirid DL. Differences in expression of heat-shock proteins and antioxidant emzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med. 1995. 1:806–820.26. Abraham NG, Lin JH, Schwartzman ML, Levere RD, Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1998. 20:543–558.
Article27. Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1β and tumor necrosis factor necrosis factor-α via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990. 276:42–44.28. Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1β-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition in isolated of the Krebs cycle enzyme aconitase. Endocrinology. 1991. 129:3167–3173.29. Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR Jr, McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1β in islets of Langerhans: evidence for a role of nitric oxide in islet dysfunction. Biochem J. 1992. 287:229–235.30. Corbett JA, Sweetland MA, Wang JL, Lancaster JR, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA. 1993. 90:1731–1735.
Article31. Eizirik DL, Welsh M, Strandell E, Welsh N, Sandler S. Interleukin-1 beta depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp 70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology. 1990. 127:2290–2297.32. Bellmann K, Wenz A, Radons J, Burkhart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995. 95:2840–2845.
Article33. Welsh N, Sandler S. Protective action by hemin against interleukin-1β induced inhibition of rat pancreatic islet function. Mol Cell Endocrinol. 1994. 103:109–114.
Article34. Margulis BA, Sandler S, Eizirik DL, Welsh N, Welsh N, Welsh M. Liposomal delivery of purified heat shock protein hsp 70 into rat pancreatic islets as protection against interleukin-1β-impaired β-cell function. Diabetes. 1991. 40:1418–1422.35. Polte T, Abate A, Dennery PA, Achroder H. Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. Arterioscler Thromb Vasc Biol. 2000. 20:1209–1215.
Article36. Farrera JA, Jauma A, Ribo JM, Peire MA, Parallada PP, Roques-Choua S, Bienvenue E, Seta P. The antioxidant role of bile pigments evaluated by chemical tests. Bioorg Med Chem. 1994. 2:181–185.
Article37. Choi MK, Alam J. Heme oxygenase-1: function, regulation and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996. 15:9–19.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Heme Oxygenase-1 on High Glucose-Induced Pancreatic beta-Cell Injury
- Oxymatrine inhibits the pyroptosis in rat insulinoma cells by affecting nuclear factor kappa B and nuclear factor (erythroidderived 2)-like 2 protein/heme oxygenase-1 pathways
- Immunohistochemical localization of heme oxygenase isozymes in the aged rat retina
- Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases