Korean J Physiol Pharmacol.
2022 May;26(3):165-174. 10.4196/kjpp.2022.26.3.165.
Oxymatrine inhibits the pyroptosis in rat insulinoma cells by affecting nuclear factor kappa B and nuclear factor (erythroidderived 2)-like 2 protein/heme oxygenase-1 pathways
- Affiliations
-
- 1Department of Pediatrics, Shanxi Medical University, Taiyuan 030001, China
- 2Pediatric Internal Medicine, Children’s Hospital of Shanxi Province, Shanxi Medical University, Taiyuan 030001, China
- KMID: 2529399
- DOI: http://doi.org/10.4196/kjpp.2022.26.3.165
Abstract
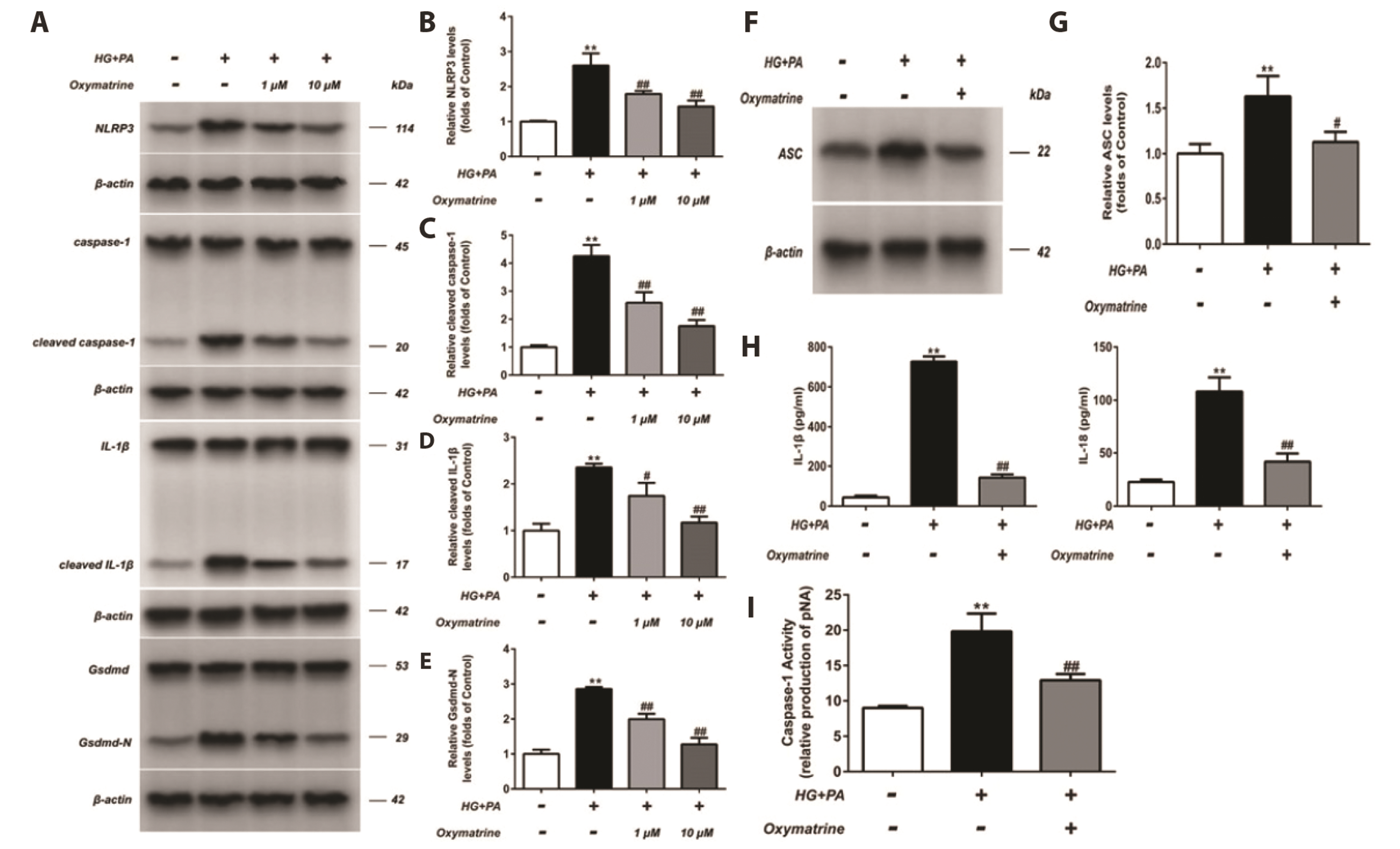
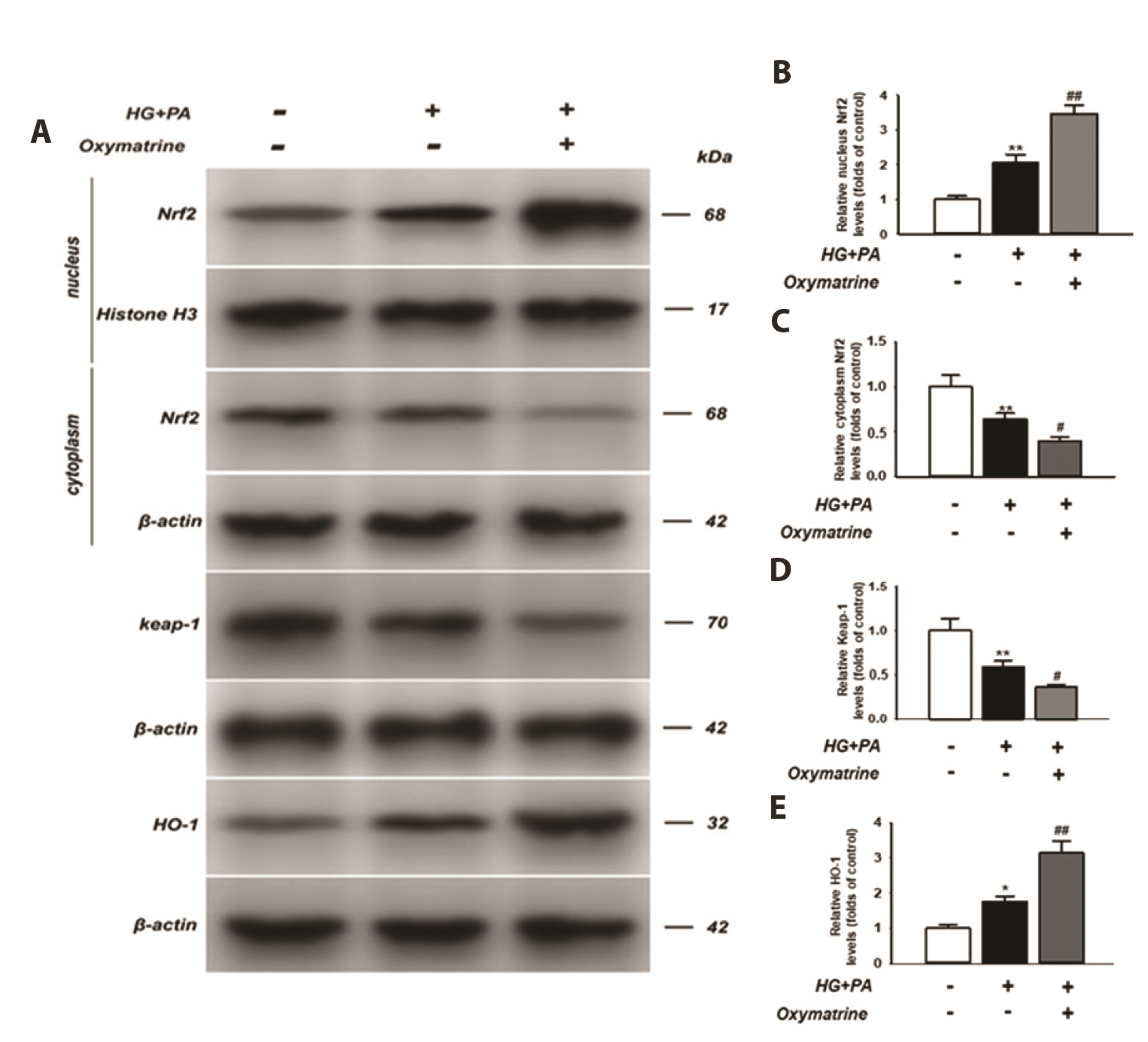
- As the mechanism underlying glucose metabolism regulation by oxymatrine is unclear, this study investigated the effects of oxymatrine on pyroptosis in INS-1 cells. Flow cytometry was employed to examine cell pyroptosis and reactive oxygen species (ROS) production. Cell pyroptosis was also investigated via transmission electron microscopy and lactate dehydrogenase (LDH) release. Protein levels were detected using western blotting and interleukin (IL)-1β and IL-18 secretion by enzyme-linked immunosorbent assay. The caspase-1 activity and DNA-binding activity of nuclear factor kappa B (NF-κB) and nuclear factor (erythroid-derived 2)-like 2 protein (Nrf2) were also assessed. In the high glucose and high fat-treated INS-1 cells (HG + PA), the caspase-1 activity and LDH content, as well as Nod-like receptor family pyrin domain containing 3, Gsdmd-N, caspase-1, apoptosis-associated specklike protein containing a CARD, IL-1β, and IL-18 levels were increased. Moreover, P65 protein levels increased in the nucleus but decreased in the cytoplasm. Oxymatrine attenuated these effects and suppressed high glucose and high fat-induced ROS production. The increased levels of nuclear Nrf2 and heme oxygenase-1 (HO-1) in the HG + PA cells were further elevated after oxymatrine treatment, whereas cytoplasmic Nrf2 and Keleh-like ECH-associated protein levels decreased. Additionally, the elevated transcriptional activity of p65 in HG + PA cells was reduced by oxymatrine, whereas that of Nrf2 increased. The results indicate that the inhibition of pyroptosis in INS-1 cells by oxymatrine, a key factor in its glucose metabolism regulation, involves the suppression of the NF-κB pathway and activation of the Nrf2/HO-1 pathway.
Keyword
Figure
Reference
-
1. Brennan MA, Cookson BT. 2000; Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 38:31–40. DOI: 10.1046/j.1365-2958.2000.02103.x. PMID: 11029688.
Article2. Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. 2014; Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunol Cell Biol. 92:314–323. DOI: 10.1038/icb.2014.4. PMID: 24492799.
Article3. Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. 2009; Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda). 24:325–331. DOI: 10.1152/physiol.00032.2009. PMID: 19996363.4. Christensen CS, Christensen DP, Lundh M, Dahllöf MS, Haase TN, Velasquez JM, Laye MJ, Mandrup-Poulsen T, Solomon TP. 2015; Skeletal muscle to pancreatic β-cell cross-talk: the effect of humoral mediators liberated by muscle contraction and acute exercise on β-cell apoptosis. J Clin Endocrinol Metab. 100:E1289–E1298. Erratum in: J Clin Endocrinol Metab. 2016;101:2265. DOI: 10.1210/jc.2014-4506. PMID: 26218753.
Article5. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. 2015; Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25:1285–1298. DOI: 10.1038/cr.2015.139. PMID: 26611636. PMCID: PMC4670995.
Article6. Schneider KS, Groß CJ, Dreier RF, Saller BS, Mishra R, Gorka O, Heilig R, Meunier E, Dick MS, Ćiković T, Sodenkamp J, Médard G, Naumann R, Ruland J, Kuster B, Broz P, Groß O. 2017; The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 21:3846–3859. DOI: 10.1016/j.celrep.2017.12.018. PMID: 29281832. PMCID: PMC5750195.
Article7. Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J. 2016; Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26:1007–1020. DOI: 10.1038/cr.2016.100. PMID: 27573174. PMCID: PMC5034106.
Article8. Masters SL, Latz E, O'Neill LA. 2011; The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 3:81ps17. DOI: 10.1126/scitranslmed.3001902. PMID: 21543720.
Article9. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. 2011; The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 17:179–188. DOI: 10.1038/nm.2279. PMID: 21217695. PMCID: PMC3076025.
Article10. Legrand-Poels S, Esser N, L'homme L, Scheen A, Paquot N, Piette J. 2014; Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochem Pharmacol. 92:131–141. DOI: 10.1016/j.bcp.2014.08.013. PMID: 25175736.
Article11. Gerber PA, Rutter GA. 2017; The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 26:501–518. DOI: 10.1089/ars.2016.6755. PMID: 27225690. PMCID: PMC5372767.
Article12. Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW. 2009; Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 30:1024–1031. DOI: 10.1093/carcin/bgp100. PMID: 19386581. PMCID: PMC2691141.
Article13. Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. 2016; Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 63:173–184. DOI: 10.1002/hep.28251. PMID: 26403645. PMCID: PMC4688087.
Article14. Jaramillo MC, Zhang DD. 2013; The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 27:2179–2191. DOI: 10.1101/gad.225680.113. PMID: 24142871. PMCID: PMC3814639.
Article15. Li S, Vaziri ND, Masuda Y, Hajighasemi-Ossareh M, Robles L, Le A, Vo K, Chan JY, Foster CE, Stamos MJ, Ichii H. 2015; Pharmacological activation of Nrf2 pathway improves pancreatic islet isolation and transplantation. Cell Transplant. 24:2273–2283. DOI: 10.3727/096368915X686210. PMID: 25581574.
Article16. Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. 2014; Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 63:605–618. DOI: 10.2337/db13-0909. PMID: 24186865.
Article17. Yi Y, Shen Y, Wu Q, Rao J, Guan S, Rao S, Huang L, Tan M, He L, Liu L, Li G, Liang S, Xiong W, Gao Y. 2018; Protective effects of oxymatrine on vascular endothelial cells from high-glucose-induced cytotoxicity by inhibiting the expression of A2B receptor. Cell Physiol Biochem. 45:558–571. DOI: 10.1159/000487033. PMID: 29402837.
Article18. Wang L, Li X, Zhang Y, Huang Y, Zhang Y, Ma Q. 2018; Oxymatrine ameliorates diabetes-induced aortic endothelial dysfunction via the regulation of eNOS and NOX4. J Cell Biochem. 120:7323–7332. DOI: 10.1002/jcb.28006. PMID: 30456880.
Article19. Guo C, Han F, Zhang C, Xiao W, Yang Z. 2014; Protective effects of oxymatrine on experimental diabetic nephropathy. Planta Med. 80:269–276. DOI: 10.1055/s-0033-1360369. PMID: 24535719.
Article20. Gao J, Xia L, Wei Y. 2021; Voltage-gated potassium channels are involved in oxymatrine-regulated islet function in rat islet β cells and INS-1 cells. Iran J Basic Med Sci. 24:460–468. DOI: 10.22038/ijbms.2021.52449.11850. PMID: 34094027. PMCID: PMC8143713. PMID: 187fce4628aa44beb16134c4845da0d6.21. Li DX, Wang CN, Wang Y, Ye CL, Jiang L, Zhu XY, Liu YJ. 2020; NLRP3 inflammasome-dependent pyroptosis and apoptosis in hippocampus neurons mediates depressive-like behavior in diabetic mice. Behav Brain Res. 391:112684. DOI: 10.1016/j.bbr.2020.112684. PMID: 32454054.
Article22. Gu J, Huang W, Zhang W, Zhao T, Gao C, Gan W, Rao M, Chen Q, Guo M, Xu Y, Xu YH. 2019; Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol. 75:105832. DOI: 10.1016/j.intimp.2019.105832. PMID: 31473434.
Article23. Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, Barazzoni R, Scherer PE, Cinti S. 2013; Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 54:2423–2436. DOI: 10.1194/jlr.M038638. PMID: 23836106. PMCID: PMC3735940.
Article24. Yang L, Liu J, Shan Q, Geng G, Shao P. 2020; High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys Res Commun. 522:471–478. Erratum in: Biochem Biophys Res Commun. 2020;528:404. DOI: 10.1016/j.bbrc.2020.05.039. PMID: 32471714.
Article25. Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F. 2014; NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 9:e104771. DOI: 10.1371/journal.pone.0104771. PMID: 25136835. PMCID: PMC4138036.
Article26. Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P. 2016; MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun. 471:423–429. DOI: 10.1016/j.bbrc.2016.02.065. PMID: 26898797. PMCID: PMC4818978.
Article27. Song Y, Yang L, Guo R, Lu N, Shi Y, Wang X. 2019; Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22. Biochem Biophys Res Commun. 509:359–366. DOI: 10.1016/j.bbrc.2018.12.139. PMID: 30591217.
Article28. Wu M, Yang Z, Zhang C, Shi Y, Han W, Song S, Mu L, Du C, Shi Y. 2021; Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism. 118:154748. DOI: 10.1016/j.metabol.2021.154748. PMID: 33675822.
Article29. Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. 2013; Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 62:194–204. DOI: 10.2337/db12-0420. PMID: 23086037. PMCID: PMC3526026.
Article30. Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. 2012; Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 7:e38285. DOI: 10.1371/journal.pone.0038285. PMID: 22701621. PMCID: PMC3372527.
Article31. Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. 2018; Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabetes Res. 2018:8193523. DOI: 10.1155/2018/8193523. PMID: 29682582. PMCID: PMC5842685.
Article32. Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. 2008; NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 105:17345–17350. DOI: 10.1073/pnas.0806198105. PMID: 18988733. PMCID: PMC2582288.
Article33. Wellen KE, Hotamisligil GS. 2005; Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119. DOI: 10.1172/JCI25102. PMID: 15864338. PMCID: PMC1087185.
Article34. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. 2009; Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 29:359–370. DOI: 10.1523/JNEUROSCI.2760-08.2009. PMID: 19144836. PMCID: PMC6664935.
Article35. Thaler JP, Schwartz MW. 2010; Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 151:4109–4115. DOI: 10.1210/en.2010-0336. PMID: 20573720. PMCID: PMC2940486.
Article36. Swisa A, Glaser B, Dor Y. 2017; Metabolic stress and compromised identity of pancreatic beta cells. Front Genet. 8:21. DOI: 10.3389/fgene.2017.00021. PMID: 28270834. PMCID: PMC5318414.
Article37. Guthrie RA, Guthrie DW. 2004; Pathophysiology of diabetes mellitus. Crit Care Nurs Q. 27:113–125. DOI: 10.1097/00002727-200404000-00003. PMID: 15137354.
Article38. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. 2020; Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 21:6275. DOI: 10.3390/ijms21176275. PMID: 32872570. PMCID: PMC7503727. PMID: 4a8a429661a346cb9eb529281e3b984f.
Article39. Liang J, Chang B, Huang M, Huang W, Ma W, Liu Y, Tai W, Long Y, Lu Y. 2018; Oxymatrine prevents synovial inflammation and migration via blocking NF-κB activation in rheumatoid fibroblast-like synoviocytes. Int Immunopharmacol. 55:105–111. DOI: 10.1016/j.intimp.2017.12.006. PMID: 29241159.
Article40. Liang L, Wu J, Luo J, Wang L, Chen ZX, Han CL, Gan TQ, Huang JA, Cai ZW. 2020; Oxymatrine reverses 5-fluorouracil resistance by inhibition of colon cancer cell epithelial-mesenchymal transition and NF-κB signaling in vitro. Oncol Lett. 19:519–526. DOI: 10.3892/ol.2019.11090. PMID: 31897166. PMCID: PMC6924048.41. Jiang G, Liu X, Wang M, Chen H, Chen Z, Qiu T. 2015; Oxymatrine ameliorates renal ischemia-reperfusion injury from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras. 30:422–429. DOI: 10.1590/S0102-865020150060000008. PMID: 26108031.
Article42. Li L, Liu Q, Fan L, Xiao W, Zhao L, Wang Y, Ye W, Lan F, Jia B, Feng H, Zhou C, Yue X, Xing G, Wang T. 2017; Protective effects of oxymatrine against arsenic trioxide-induced liver injury. Oncotarget. 8:12792–12799. DOI: 10.18632/oncotarget.12478. PMID: 27713174. PMCID: PMC5355055.
Article43. Li M, Zhang X, Cui L, Yang R, Wang L, Liu L, Du W. 2011; The neuroprotection of oxymatrine in cerebral ischemia/reperfusion is related to nuclear factor erythroid 2-related factor 2 (nrf2)-mediated antioxidant response: role of nrf2 and hemeoxygenase-1 expression. Biol Pharm Bull. 34:595–601. DOI: 10.1248/bpb.34.595. PMID: 21532144.
Article44. Xu J, Li C, Li Z, Yang C, Lei L, Ren W, Su Y, Chen C. 2018; Protective effects of oxymatrine against lipopolysaccharide/D-galactosamine-induced acute liver failure through oxidative damage, via activation of Nrf2/HO-1 and modulation of inflammatory TLR4-signaling pathways. Mol Med Rep. 17:1907–1912. DOI: 10.3892/mmr.2017.8060.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-κB Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells
- Immunohistochemical localization of heme oxygenase isozymes in the aged rat retina
- Heme Oxygenase-1 is Involved in the Down-regulation of Nuclear Transcription Factor kappa B Activation in the Colonic Epithelium During Inflammation
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells