Anat Cell Biol.
2015 Jun;48(2):95-103. 10.5115/acb.2015.48.2.95.
A single fraction from Uncaria sinensis exerts neuroprotective effects against glutamate-induced neurotoxicity in primary cultured cortical neurons
- Affiliations
-
- 1Department of Korean Medicine, School of Korean Medicine, Pusan National University, Yangsan, Korea. choibt@pusan.ac.kr
- 2Division of Meridian and Structural Medicine, School of Korean Medicine, Pusan National University, Yangsan, Korea.
- KMID: 1845277
- DOI: http://doi.org/10.5115/acb.2015.48.2.95
Abstract
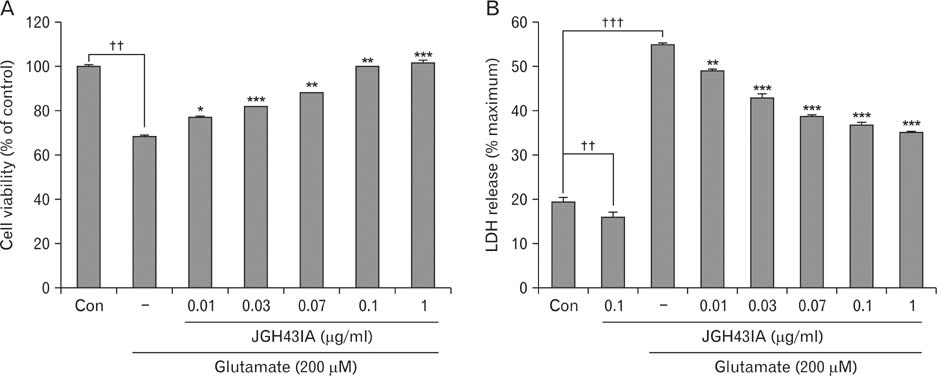
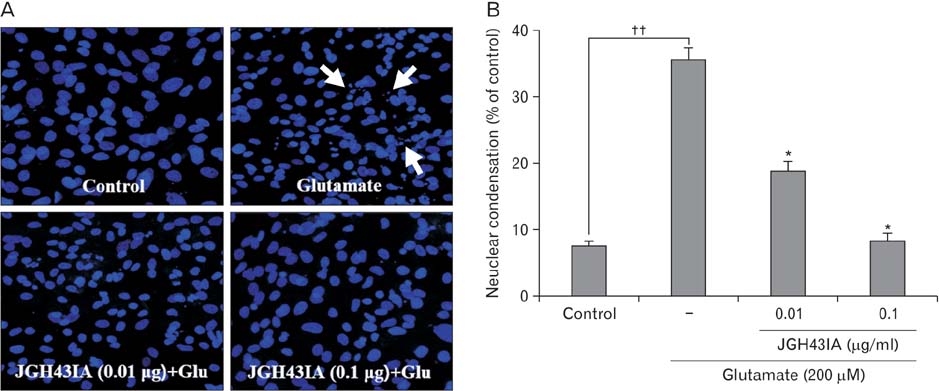
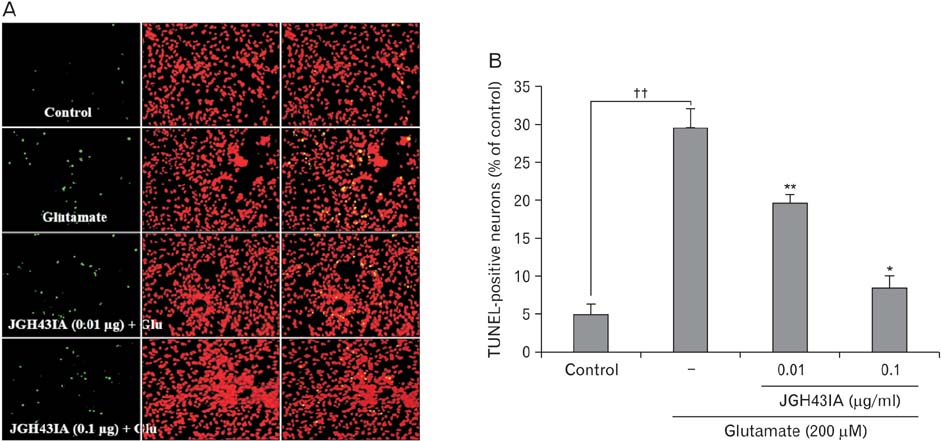
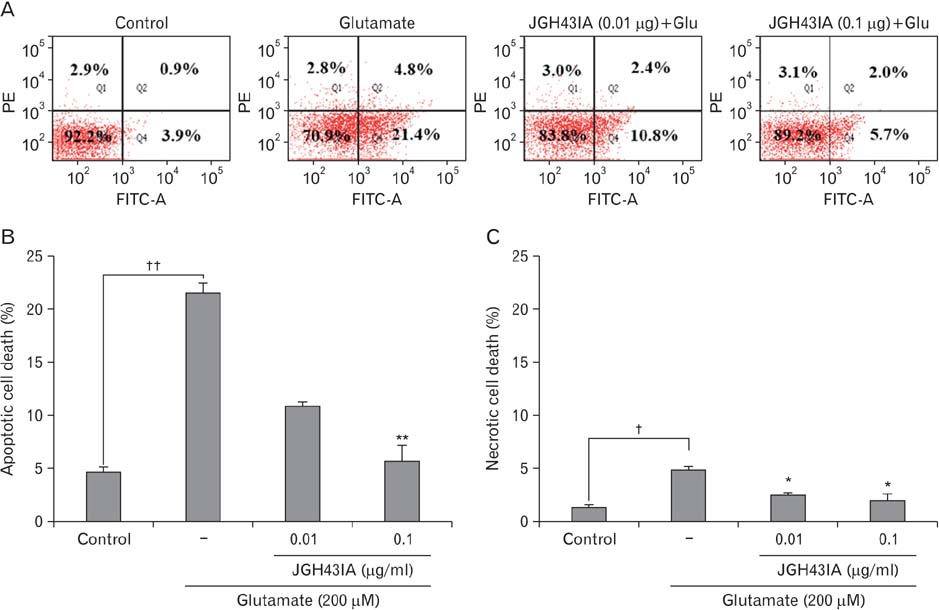
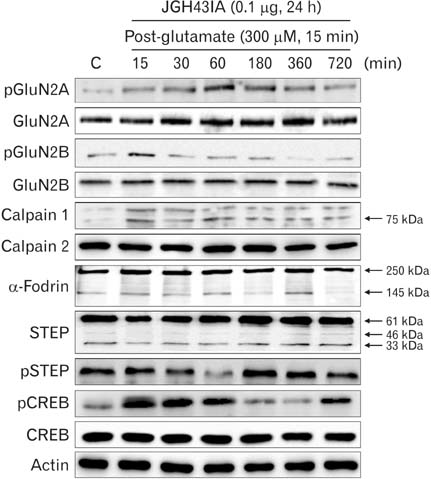
- We identified a neuroprotective single fraction among 62 ones of hexane extract from Uncaria sinensis (JGH43IA) and investigated its effects and mechanisms in primary cortical neurons. Pretreatment with JGH43IA showed a significantly increase cell viability in a dose-dependent manner with a decrease in the lactate dehydrogenase release. When we performed morphological assay and flow cytometry to determination of the type of cell death, pretreatment with JGH43IA showed a significant reduction of glutamate-induced apoptotic cell death. Then we explored the downstream signaling pathways of N-methyl-D-aspartate receptor (NMDAR) with calpain activation to elucidate possible pathways of neuroprotection by JGH43IA. Pretreatment with JGH43IA exhibited a significant attenuation of NMDAR GluN2B subunit activation and a decrease in active form of calpain 1 leading to subsequent cleavage of striatal-enriched protein tyrosine phosphatase (STEP). In addition, pretreatment with JGH43IA showed a marked increase of cAMP responsive element binding protein. These results suggest that JGH43IA may have neuroprotective effects through down-regulation of NMDAR GluN2B subunit and calpain 1 activation, and subsequent alleviation of STEP cleavage. This single fraction from U. sinensis might be a useful therapeutic agent for brain disorder associated with glutamate injury.
MeSH Terms
-
Brain Diseases
Calpain
Carrier Proteins
Cell Death
Cell Survival
Down-Regulation
Flow Cytometry
Glutamates
Glutamic Acid
L-Lactate Dehydrogenase
N-Methylaspartate
Neurons*
Neuroprotective Agents*
Protein Tyrosine Phosphatases
Receptors, N-Methyl-D-Aspartate
Uncaria*
Calpain
Carrier Proteins
Glutamates
Glutamic Acid
L-Lactate Dehydrogenase
N-Methylaspartate
Neuroprotective Agents
Protein Tyrosine Phosphatases
Receptors, N-Methyl-D-Aspartate
Figure
Reference
-
1. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000; 130:4S Suppl. 1007S–1015S.2. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993; 262:689–695.3. Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001; 69:369–381.4. Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009; 617:1–11.5. Liu L, Zhang R, Liu K, Zhou H, Tang Y, Su J, Yu X, Yang X, Tang M, Dong Q. Tissue kallikrein alleviates glutamate-induced neurotoxicity by activating ERK1. J Neurosci Res. 2009; 87:3576–3590.6. Xu B, Xu ZF, Deng Y. Protective effects of MK-801 on manganese-induced glutamate metabolism disorder in rat striatum. Exp Toxicol Pathol. 2010; 62:381–390.7. Lipton SA. Redox sensitivity of NMDA receptors. Methods Mol Biol. 1999; 128:121–130.8. Volbracht C, Chua BT, Ng CP, Bahr BA, Hong W, Li P. The critical role of calpain versus caspase activation in excitotoxic injury induced by nitric oxide. J Neurochem. 2005; 93:1280–1292.9. Poddar R, Deb I, Mukherjee S, Paul S. NR2B-NMDA receptor mediated modulation of the tyrosine phosphatase STEP regulates glutamate induced neuronal cell death. J Neurochem. 2010; 115:1350–1362.10. Gladding CM, Sepers MD, Xu J, Zhang LY, Milnerwood AJ, Lombroso PJ, Raymond LA. Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington's disease mouse model. Hum Mol Genet. 2012; 21:3739–3752.11. Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009; 29:9330–9343.12. Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003; 6:34–42.13. Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005; 8:1051–1058.14. Shimada Y, Goto H, Kogure T, Shibahara N, Sakakibara I, Sasaki H, Terasawa K. Protective effect of phenolic compounds isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death. Am J Chin Med. 2001; 29:173–180.15. Shimada Y, Yokoyama K, Goto H, Sekiya N, Mantani N, Tahara E, Hikiami H, Terasawa K. Protective effect of keishi-bukuryo-gan and its constituent medicinal plants against nitric oxide donor-induced neuronal death in cultured cerebellar granule cells. Phytomedicine. 2004; 11:404–410.16. Watanabe H, Zhao Q, Matsumoto K, Tohda M, Murakami Y, Zhang SH, Kang TH, Mahakunakorn P, Maruyama Y, Sakakibara I, Aimi N, Takayama H. Pharmacological evidence for antidementia effect of Choto-san (Gouteng-san), a traditional Kampo medicine. Pharmacol Biochem Behav. 2003; 75:635–643.17. Tan SN, Yong JW, Teo CC, Ge L, Chan YW, Hew CS. Determination of metabolites in Uncaria sinensis by HPLC and GC-MS after green solvent microwave-assisted extraction. Talanta. 2011; 83:891–898.18. Jang JY, Kim HN, Kim YR, Hong JW, Choi YW, Choi YH, Shin HK, Choi BT. Hexane extract from Uncaria sinensis exhibits anti-apoptotic properties against glutamate-induced neurotoxicity in primary cultured cortical neurons. Int J Mol Med. 2012; 30:1465–1472.19. Park SH, Kim JH, Park SJ, Bae SS, Choi YW, Hong JW, Choi BT, Shin HK. Protective effect of hexane extracts of Uncaria sinensis against photothrombotic ischemic injury in mice. J Ethnopharmacol. 2011; 138:774–779.20. Jang JY, Choi YW, Kim HN, Kim YR, Hong JW, Bae DW, Park SJ, Shin HK, Choi BT. Neuroprotective effects of a novel single compound 1-methoxyoctadecan-1-ol isolated from Uncaria sinensis in primary cortical neurons and a photothrombotic ischemia model. PLoS One. 2014; 9:e85322.21. Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, Terasawa K. Evaluation of the protective effects of alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J Pharm Pharmacol. 1999; 51:715–722.22. Zhou J, Zhou S. Antihypertensive and neuroprotective activities of rhynchophylline: the role of rhynchophylline in neurotransmission and ion channel activity. J Ethnopharmacol. 2010; 132:15–27.23. Bittigau P, Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol. 1997; 12:471–485.24. Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010; 460:525–542.25. Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995; 15:961–973.26. Nicotera P, Ankarcrona M, Bonfoco E, Orrenius S, Lipton SA. Neuronal necrosis and apoptosis: two distinct events induced by exposure to glutamate or oxidative stress. Adv Neurol. 1997; 72:95–101.27. Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010; 11:682–696.28. Qiu S, Li XY, Zhuo M. Post-translational modification of NMDA receptor GluN2B subunit and its roles in chronic pain and memory. Semin Cell Dev Biol. 2011; 22:521–529.29. Hu B, Friberg H, Wieloch T. Protracted tyrosine phosphorylation of the glutamate receptor subunit NR2 in the rat hippocampus following transient cerebral ischemia is prevented by intra-ischemic hypothermia. Ther Hypothermia Temp Manag. 2011; 1:159–164.30. Herrera F, Martin V, García-Santos G, Rodriguez-Blanco J, Antolín I, Rodriguez C. Melatonin prevents glutamate-induced oxytosis in the HT22 mouse hippocampal cell line through an antioxidant effect specifically targeting mitochondria. J Neurochem. 2007; 100:736–746.31. Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998; 141:1423–1432.32. Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012; 64:65–87.33. Elphick LM, Hawat M, Toms NJ, Meinander A, Mikhailov A, Eriksson JE, Kass GE. Opposing roles for caspase and calpain death proteases in L-glutamate-induced oxidative neurotoxicity. Toxicol Appl Pharmacol. 2008; 232:258–267.34. Wang Y, Briz V, Chishti A, Bi X, Baudry M. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci. 2013; 33:18880–18892.35. Deb I, Das S. Thyroid hormones protect astrocytes from morphine-induced apoptosis by regulating nitric oxide and pERK 1/2 pathways. Neurochem Int. 2011; 58:861–871.36. Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J Neurosci. 2001; 21:4125–4133.37. Gao J, Siddoway B, Huang Q, Xia H. Inactivation of CREB mediated gene transcription by HDAC8 bound protein phosphatase. Biochem Biophys Res Commun. 2009; 379:1–5.38. Lee E, Sidoryk-Wêgrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012; 287:26817–26828.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuroprotective Potential of Cyanidin-3-glucoside Fraction Extracted from Mulberry Following Oxygen-glucose Deprivation
- Effect of Iron-chelator on the Neurotoxicity Induced by Oxygen Radicals
- Study on the Effect of Vitamin E Against Methylmercury-induced Neurotoxicity in Cultured Spinal Motor Neurons
- Effect of Oxygen Radicals on Cultured Cerebral Neurons of Neonatal Mouse
- Antioxidant Effect of Allopurinol on Oxygen Radical-Induced Toxicity in Cultured Mouse Cerebral Neurons