J Korean Soc Transplant.
2013 Dec;27(4):174-184. 10.4285/jkstn.2013.27.4.174.
Effects of Human Adipose-Tissue Derived Stem Cell Infusion on the Immunological Consequences in Skin Allograft Mice
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea. sejoonkim@hanmail.net
- 2Department of Pathology, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea.
- 3Department of Anesthesiology, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea.
- KMID: 1841126
- DOI: http://doi.org/10.4285/jkstn.2013.27.4.174
Abstract
- BACKGROUND
Many in vitro experiments have demonstrated the immunosuppressive properties of mesenchymal stem cells (MSCs). However, such properties have not yet been fully established in an in vivo setting. The purpose of this study was to determine immunosuppressive and anti-inflammatory properties of MSCs in a preclinical animal model in order to pave the way for replacement of conventional immunosuppressive therapy.
METHODS
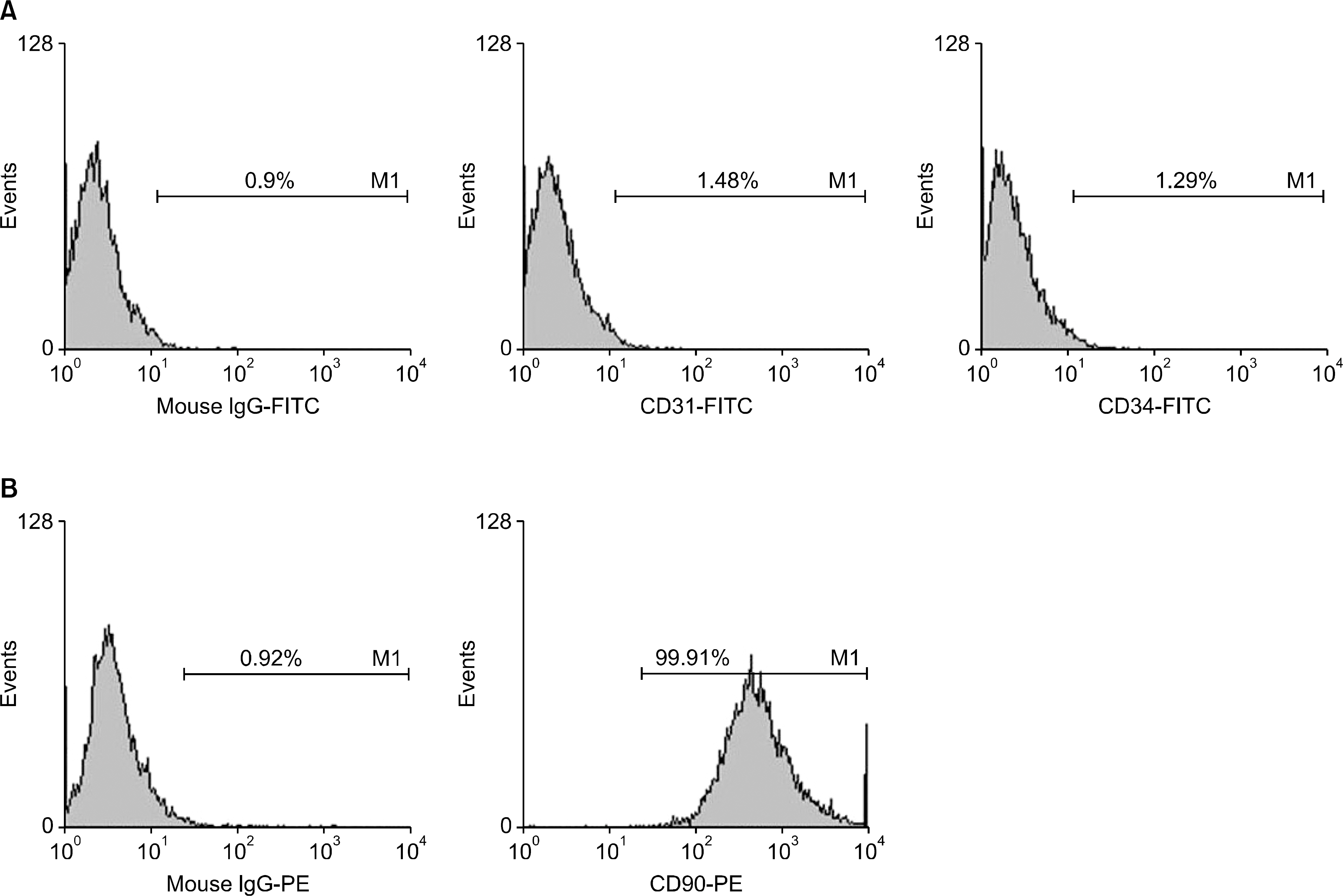
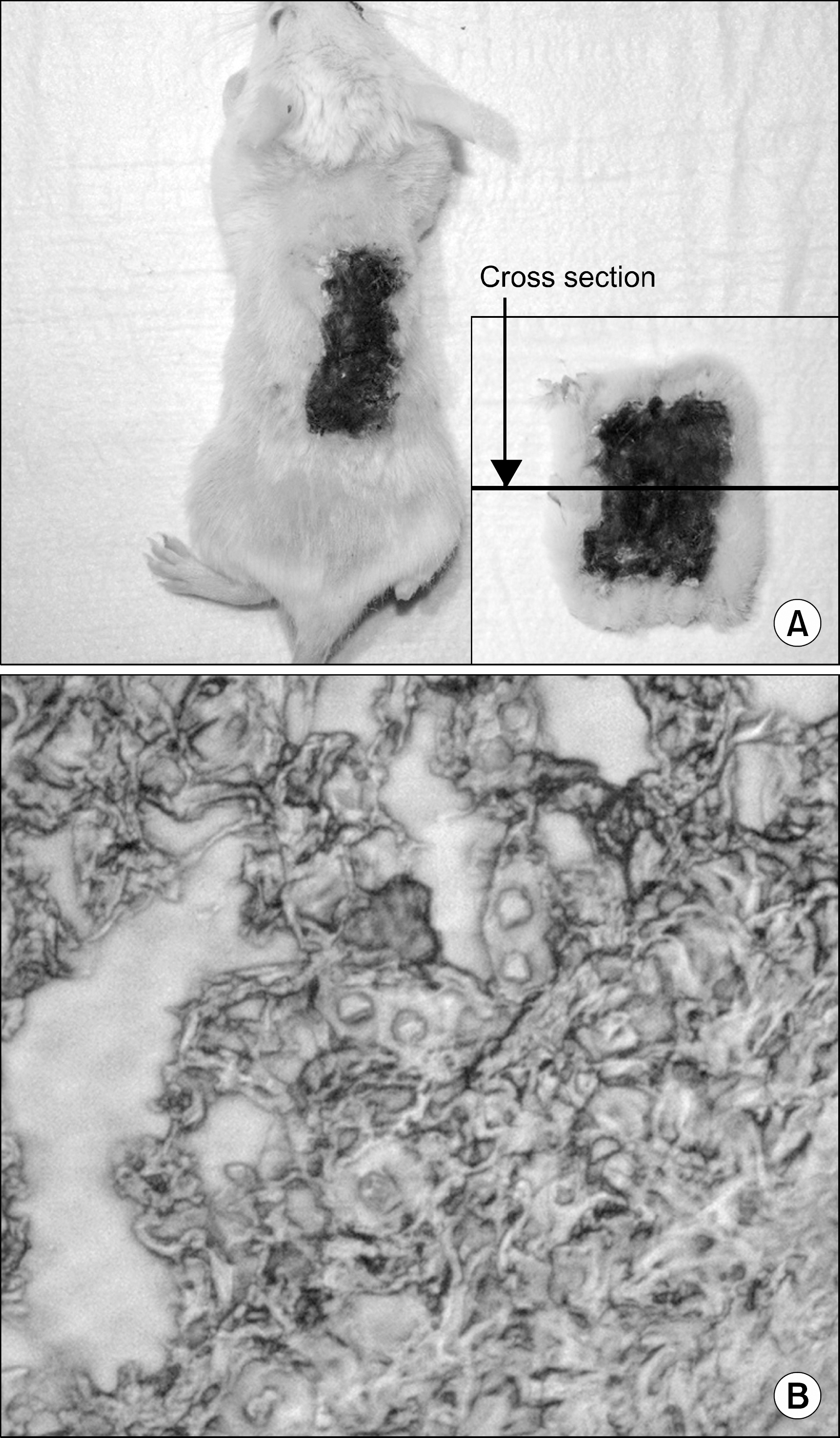
Male C57BL/6 mice and male BALB/c mice were chosen as skin graft donors and recipients, respectively. After performance of full-thickness skin transplantation on the back of mice, adipose tissue derived stem cells (1.0x10(6)/0.1 mL) stained with 4, 6-diamidino-2-phenylindole were transplanted into adipose tissue derived stem cell (ASC)-infused mice and phosphate buffered saline (PBS; 0.1 mL) was infused into PBS-infused mice. Immunological properties and graft survival were accessed and compared.
RESULTS
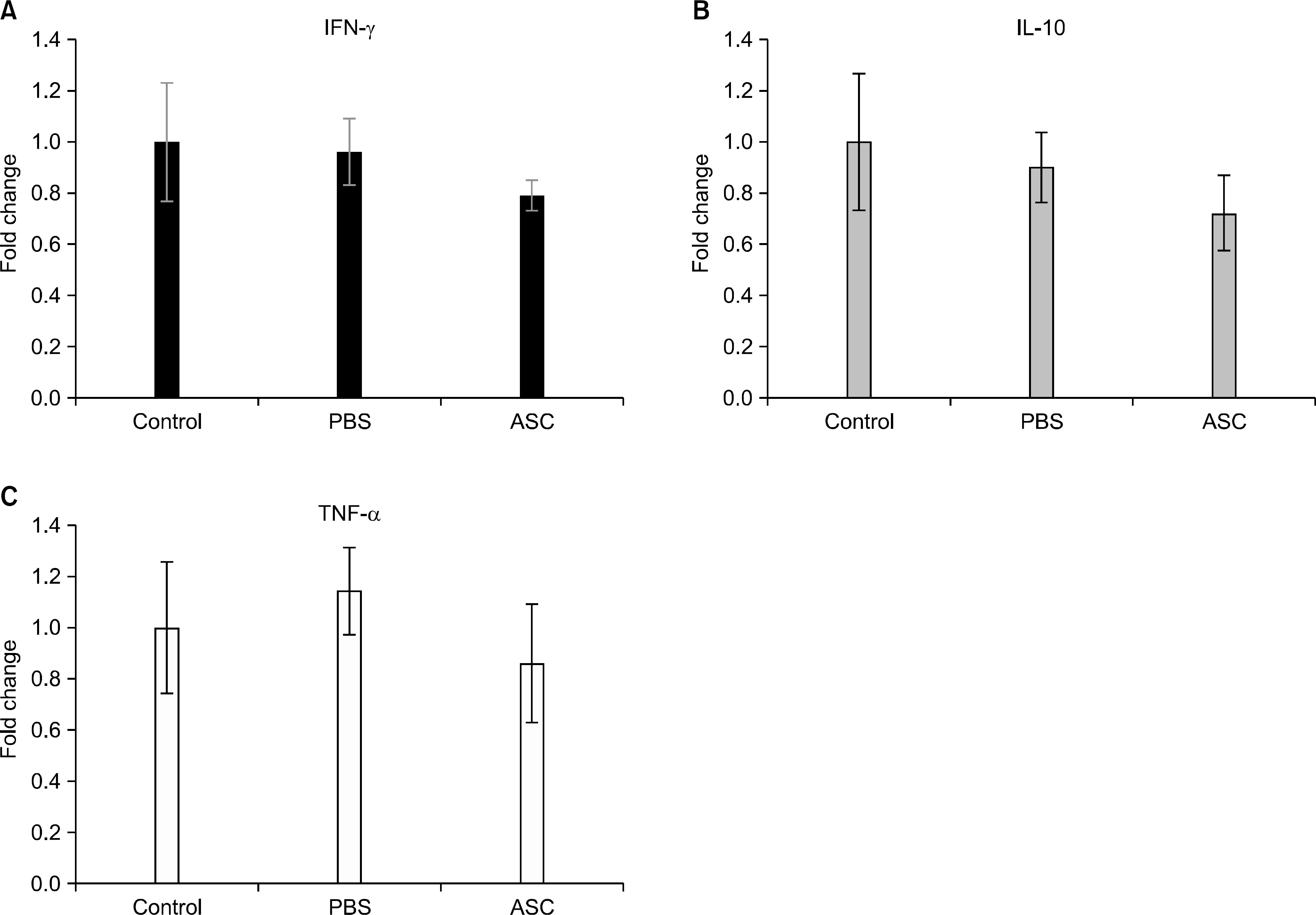
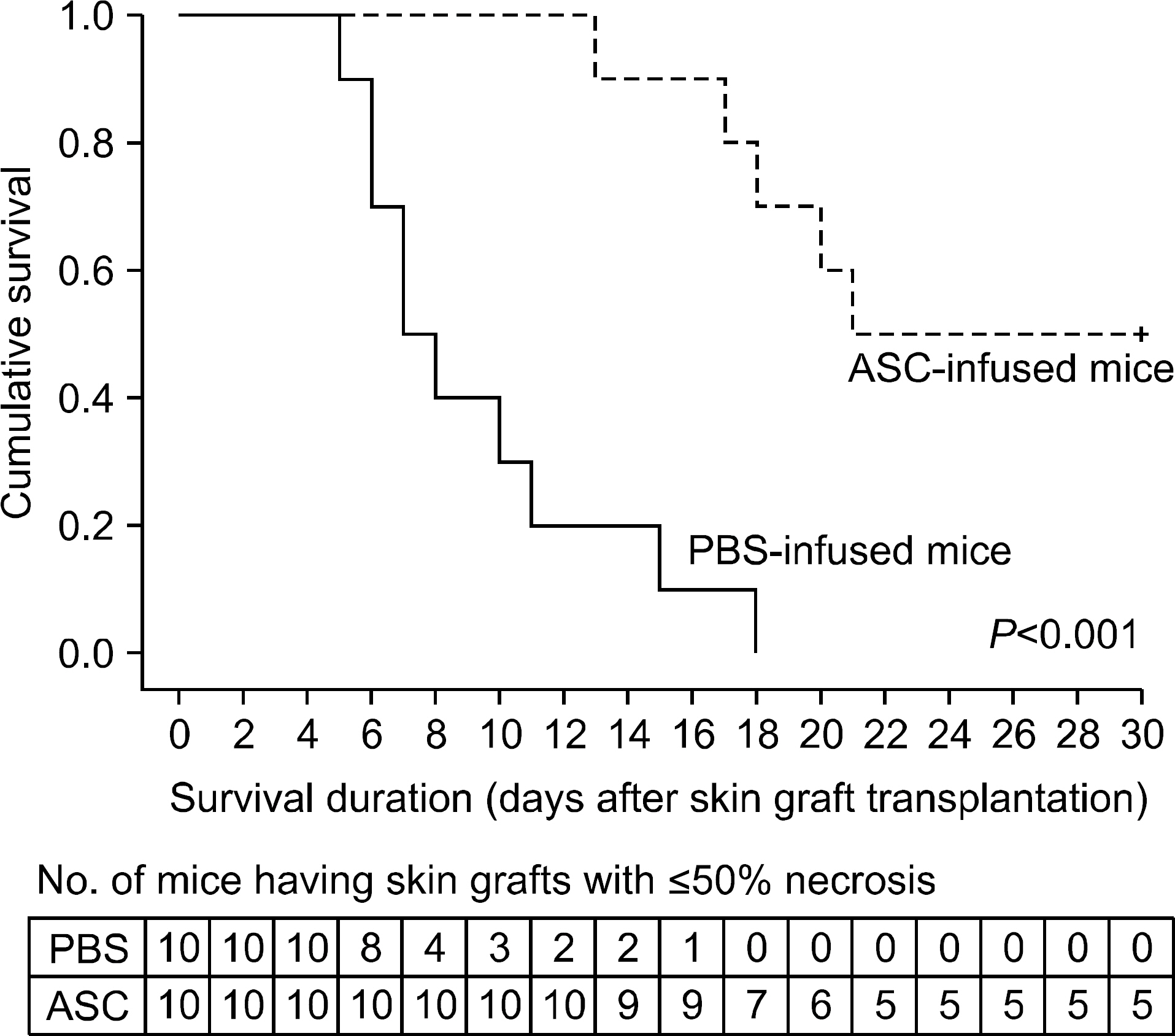
The serum levels of proinflammatory interleukin (IL)-6 showed a decrease in ASC-infused mice compared to PBS-infused mice (P<0.005). In addition, interferon-lambda, IL-10, and tumor necrosis factor-alpha mRNA levels in the skin graft showed a decrease in ASC-infused mice, although without statistical significance. In ASC-infused mice, donor specific hyporesponsiveness was identified in a mixed lymphocyte reaction assay at 30 days after transplantation. In addition, ASC-infusion resulted in markedly prolonged skin allograft survival compared with PBS-infusion (P<0.001).
CONCLUSIONS
Administration of ASC not only induced anti-inflammation and immunosuppression, but also resulted in prolonged graft survival, suggestive of their potent immunosuppressive properties. Therefore, conduct of further and more exquisite studies will be required in order to determine the role of MSC in the solid organ transplantation field in order to avoid adverse effects and toxicities caused by chemical immunosuppressive regimens.
Keyword
MeSH Terms
-
Adipose Tissue
Animals
Graft Survival
Humans*
Immunosuppression
Interleukin-10
Interleukins
Lymphocyte Culture Test, Mixed
Male
Mesenchymal Stromal Cells
Mice*
Models, Animal
Organ Transplantation
RNA, Messenger
Skin Transplantation
Skin*
Stem Cells*
Tissue Donors
Transplantation, Homologous*
Transplants
Tumor Necrosis Factor-alpha
Interleukin-10
Interleukins
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1). Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004; 13:117–30.
Article2). Anam K, Amare MF, Zins SR, Davis TA. Infusion of Lin- bone marrow cells results in multilineage macro-chimerism and skin allograft tolerance in minimally con-ditioned recipient mice. Transpl Immunol. 2010; 24:69–75.
Article3). Bartholomew A, Patil S, Mackay A, Nelson M, Buyaner D, Hardy W, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001; 12:1527–41.4). Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002; 30:42–8.
Article5). Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–43.
Article6). Hettiaratchy S, Melendy E, Randolph MA, Coburn RC, Neville DM Jr, Sachs DH, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004; 77:514–21.7). Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008; 121:50–8.
Article8). Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxyge-nase-mediated tryptophan degradation. Blood. 2004; 103:4619–21.
Article9). Petit F, Minns AB, Nazzal JA, Hettiaratchy SP, Lantieri LA, Randolph MA, et al. Prolongation of skin allograft survival after neonatal injection of donor bone marrow and epidermal cells. Plast Reconstr Surg. 2004; 113:270–6.
Article10). Siemionow M, Demir Y, Mukherjee A, Klimczak A. Development and maintenance of donor-specific chimer-ism in semi-allogenic and fully major histocompatibility complex mismatched facial allograft transplants. Transplantation. 2005; 79:558–67.
Article11). Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–97.
Article12). Wang Y, Liu J, Xu C, Zhang W, Bai L, Li N, et al. Bone marrow transplantation combined with mesenchymal stem cells induces immune tolerance without cytotoxic con-ditioning. J Surg Res. 2011; 171:e123–31.
Article13). Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I, et al. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J Plast Reconstr Aesthet Surg. 2011; 64:1647–56.
Article14). Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008; 15:109–16.
Article15). Trento C, Dazzi F. Mesenchymal stem cells and innate tolerance: biology and clinical applications. Swiss Med Wkly. 2010; 140:w13121.
Article16). Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–22.
Article17). Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012; 1:200–5.
Article18). Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006; 81:1589–95.
Article19). Kim SJ, Park KC, Lee JU, Kim KJ, Kim DG. Therapeu-tic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes. J Korean Surg Soc. 2011; 81:176–86.
Article20). Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF se-creted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011; 6:e17899.
Article21). Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in is-chemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005; 25:2542–7.
Article22). Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I, et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res. 2008; 300:115–24.
Article23). Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007; 262:509–25.
Article24). Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–506.
Article25). Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107:367–72.
Article26). Friedenstein AJ, Chailakhjan RK, Lalykina KS. The de-velopment of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970; 3:393–403.
Article27). Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and mono-cyte-derived dendritic cells. J Immunol. 2006; 177:2080–7.
Article28). Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adi-pose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009; 17:540–7.
Article29). Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cyto-therapy. 2006; 8:315–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy
- Effects of Platelet-Rich Plasma, Adipose-Derived Stem Cells, and Stromal Vascular Fraction on the Survival of Human Transplanted Adipose Tissue