J Korean Med Sci.
2014 Nov;29(Suppl 3):S193-S200. 10.3346/jkms.2014.29.S3.S193.
Effects of Platelet-Rich Plasma, Adipose-Derived Stem Cells, and Stromal Vascular Fraction on the Survival of Human Transplanted Adipose Tissue
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Korea University College of Medicine, Seoul, Korea. yesanam2@korea.ac.kr
- 2Medical Science Research Center, Ansan Hospital, Korea University Medical Center, Ansan, Korea.
- KMID: 2151413
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S193
Abstract
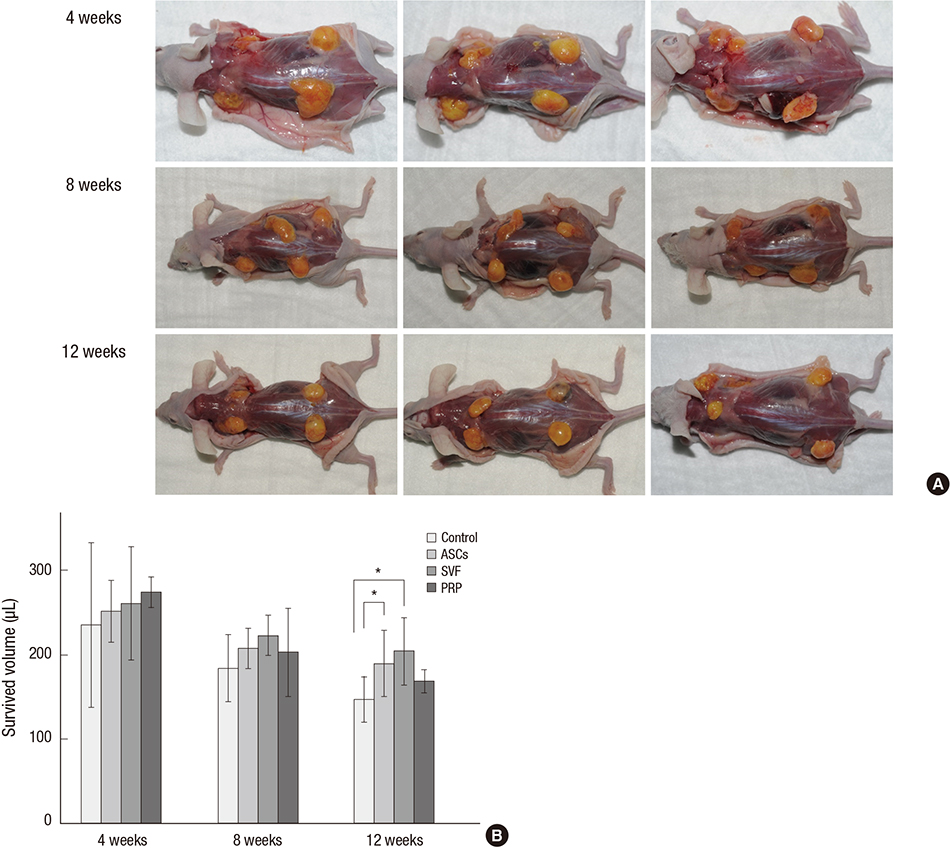
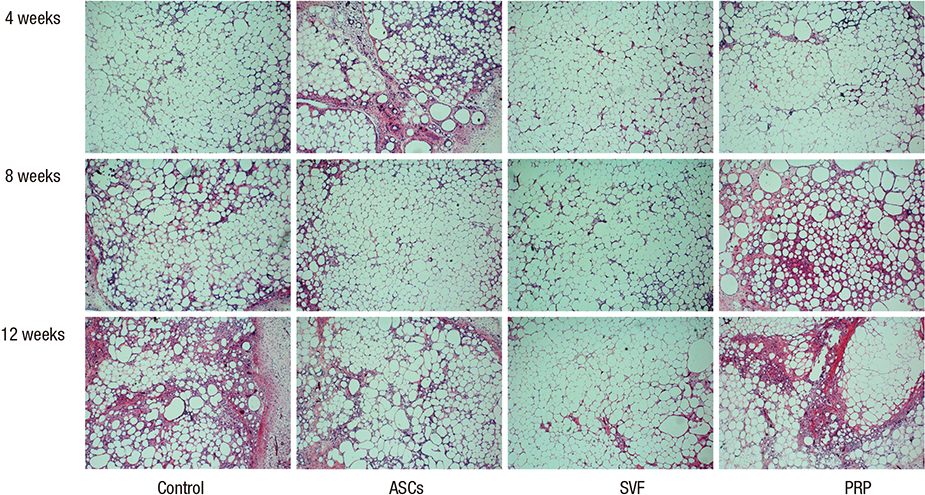
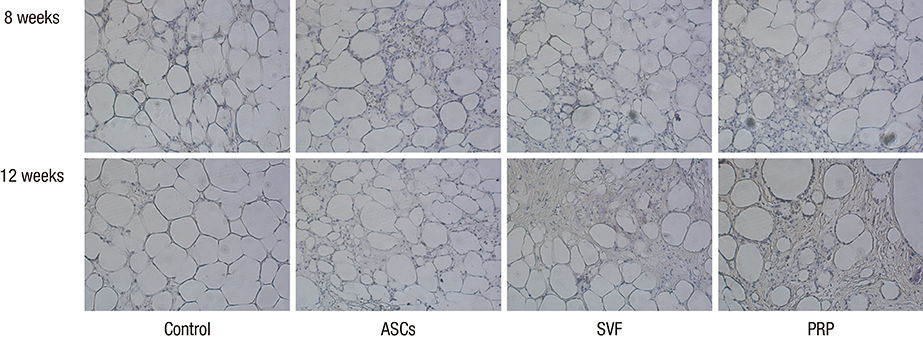
- Traditional adipose tissue transplantation has unpredictable viability and poor absorption rates. Recent studies have reported that treatment with platelet-rich plasma (PRP), adipose-derived stem cells (ASCs), and stromal vascular fraction (SVF) are related to increased survival of grafted adipose tissue. This study was the first simultaneous comparison of graft survival in combination with PRP, ASCs, and SVF. Adipose tissues were mixed with each other, injected subcutaneously into the back of nude mice, and evaluated at 4, 8, and 12 weeks. Human adipocytes were grossly maintained in the ASCs and SVF mixtures. Survival of the adipose tissues with PRP was observed at 4 weeks and with SVF at 8 and 12 weeks. At 12 weeks, volume reduction in the ASCs and SVF mixtures were 36.9% and 32.1%, respectively, which were significantly different from that of the control group without adjuvant treatment, 51.0%. Neovascular structures were rarely observed in any of the groups. Our results suggest that the technique of adding ASCs or SVF to transplanted adipose tissue might be more effective than the conventional grafting method. An autologous adipose tissue graft in combination with ASCs or SVF may potentially contribute to stabilization of engraftment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy and Safety of Autologous Stromal Vascular Fraction in the Treatment of Empty Nose Syndrome
Do-Youn Kim, Hye Ran Hong, Eun Wook Choi, Sang Won Yoon, Yong Ju Jang
Clin Exp Otorhinolaryngol. 2018;11(4):281-287. doi: 10.21053/ceo.2017.01634.
Reference
-
1. Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000; 26:1159–1166.2. Illouz YG. Present results of fat injection. Aesthetic Plast Surg. 1988; 12:175–181.3. Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006; 118:121s–128s.4. Ramon Y, Shoshani O, Peled IJ, Gilhar A, Carmi N, Fodor L, Risin Y, Ullmann Y. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast Reconstr Surg. 2005; 115:197–201. discsussion 2-3.5. Moore JH Jr, Kolaczynski JW, Morales LM, Considine RV, Pietrzkowski Z, Noto PF, Caro JF. Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg. 1995; 19:335–339.6. Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001; 27:819–826.7. Pu LL, Cui X, Fink BF, Cibull ML, Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann Plast Surg. 2005; 54:288–292.8. Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997; 55:1294–1299.9. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.10. Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, Chung HY, Cho BC, Byun JS. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013; 40:496–504.11. Kim YJ, Jeong JH. Clinical application of adipose stem cells in plastic surgery. J Korean Med Sci. 2014; 29:462–467.12. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228.13. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001; 189:54–63.14. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.15. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.16. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109:1292–1298.17. You HJ, Han SK. Cell therapy for wound healing. J Korean Med Sci. 2014; 29:311–319.18. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013; 15:641–648.19. Eun SC. Composite tissue allotransplantation immunology. Arch Plast Surg. 2013; 40:141–153.20. Smith P, Adams WP Jr, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006; 117:1836–1844.21. Yun JH, Yoo JH, Choi SH, Lee MH, Lee SJ, Song SU, Oh NS. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration of calvarial defects in rabbits. Tissue Eng Regen Med. 2012; 9:17–23.22. Liu M, Guo L, Liu Y, Pei Y, Li N, Jin M, Ma L, Li Z, Sun B, Li C. Adipose stromal-vascular fraction-derived paracrine factors regulate adipogenesis. Mol Cell Biochem. 2014; 385:115–123.23. Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013; 19:1327–1335.24. Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013; 8:e80364.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brief Review of Adipose Derived Cells
- Fat grafts enriched with adipose-derived stem cells
- The Efficacy and Safety of Platelet-Rich Plasma and Adipose-Derived Stem Cells: An Update
- A Novel Hypothesis and Characterization to Isolate Microvascular Endothelial Cells Simultaneously with AdiposeDerived Stem Cells from the Human Adipose-Derived Stromal Vascular Fraction
- Differentiation of Human Adult Adipose Derived Stem Cell in vitro and Immunohistochemical Study of Adipose Derived Stem Cell after Intracerebral Transplantation in Rats