Korean J Physiol Pharmacol.
2008 Aug;12(4):199-204. 10.4196/kjpp.2008.12.4.199.
Sorting Nexin 17 Interacts Directly with Kinesin Superfamily KIF1B beta Protein
- Affiliations
-
- 1Department of Biochemistry, National Research Laboratory for Mitochondrial Signaling, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan, Korea. daehyun@inje.ac.kr
- 2Department of Physiology, National Research Laboratory for Mitochondrial Signaling, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan, Korea.
- KMID: 1838359
- DOI: http://doi.org/10.4196/kjpp.2008.12.4.199
Abstract
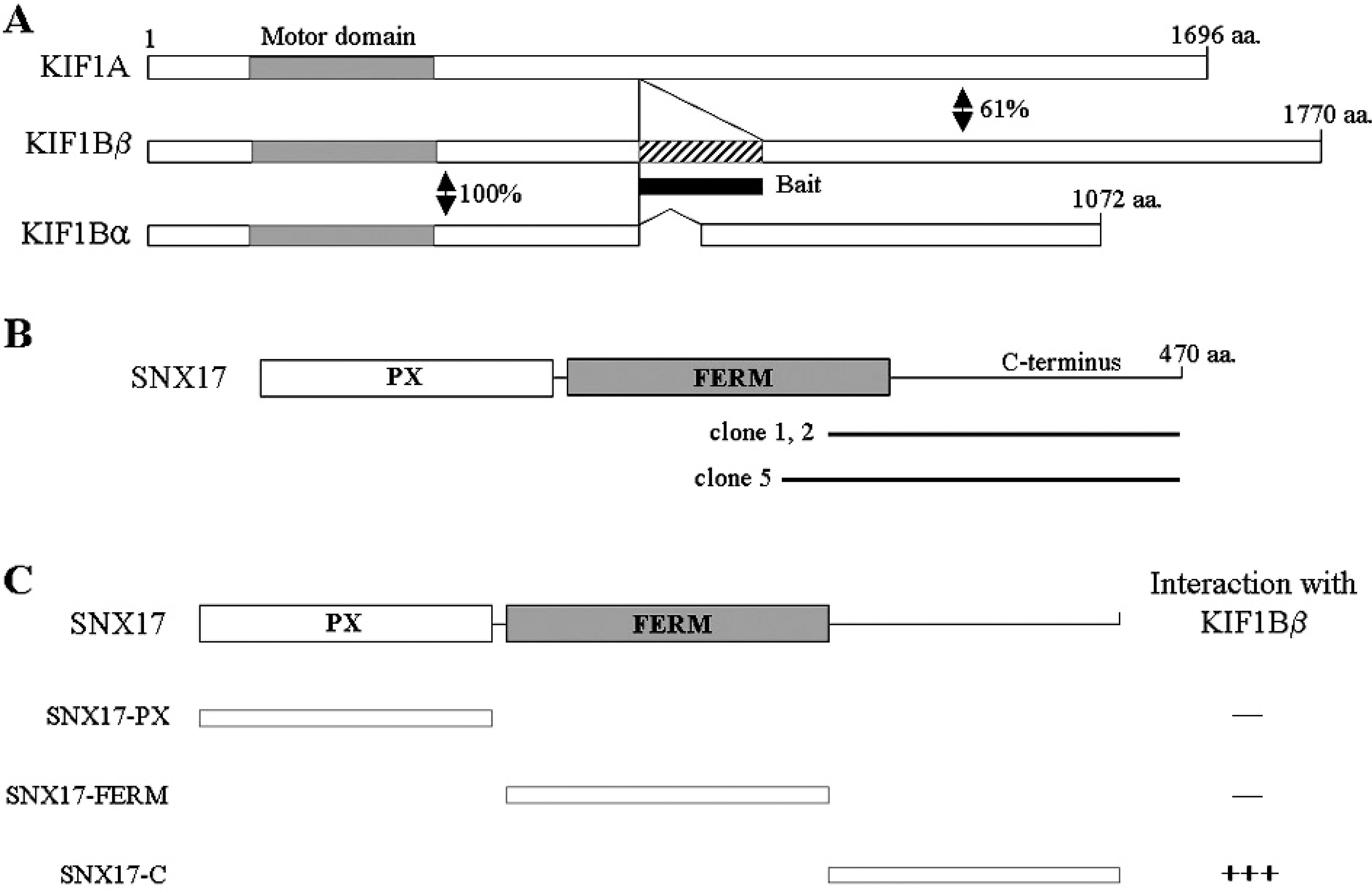
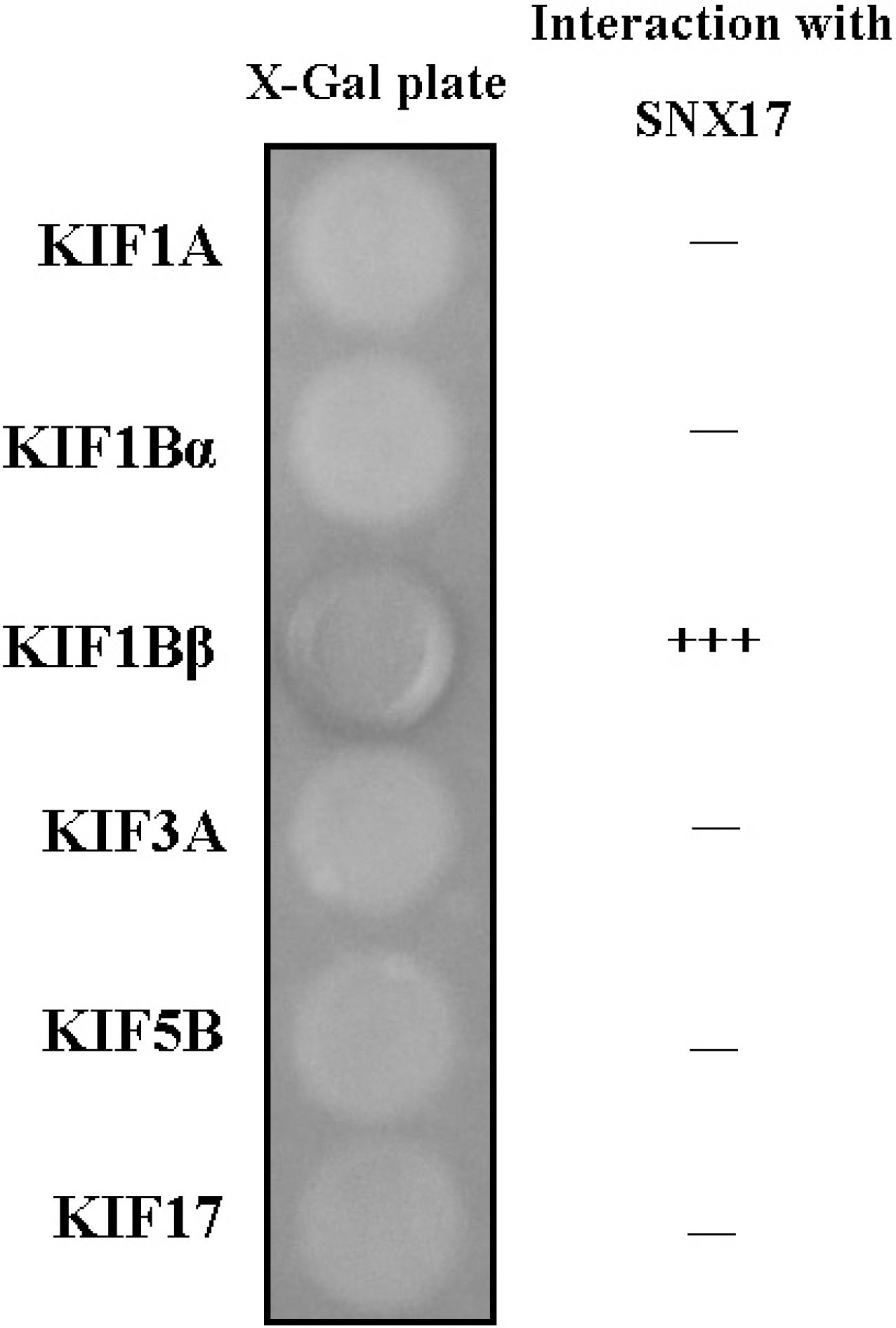
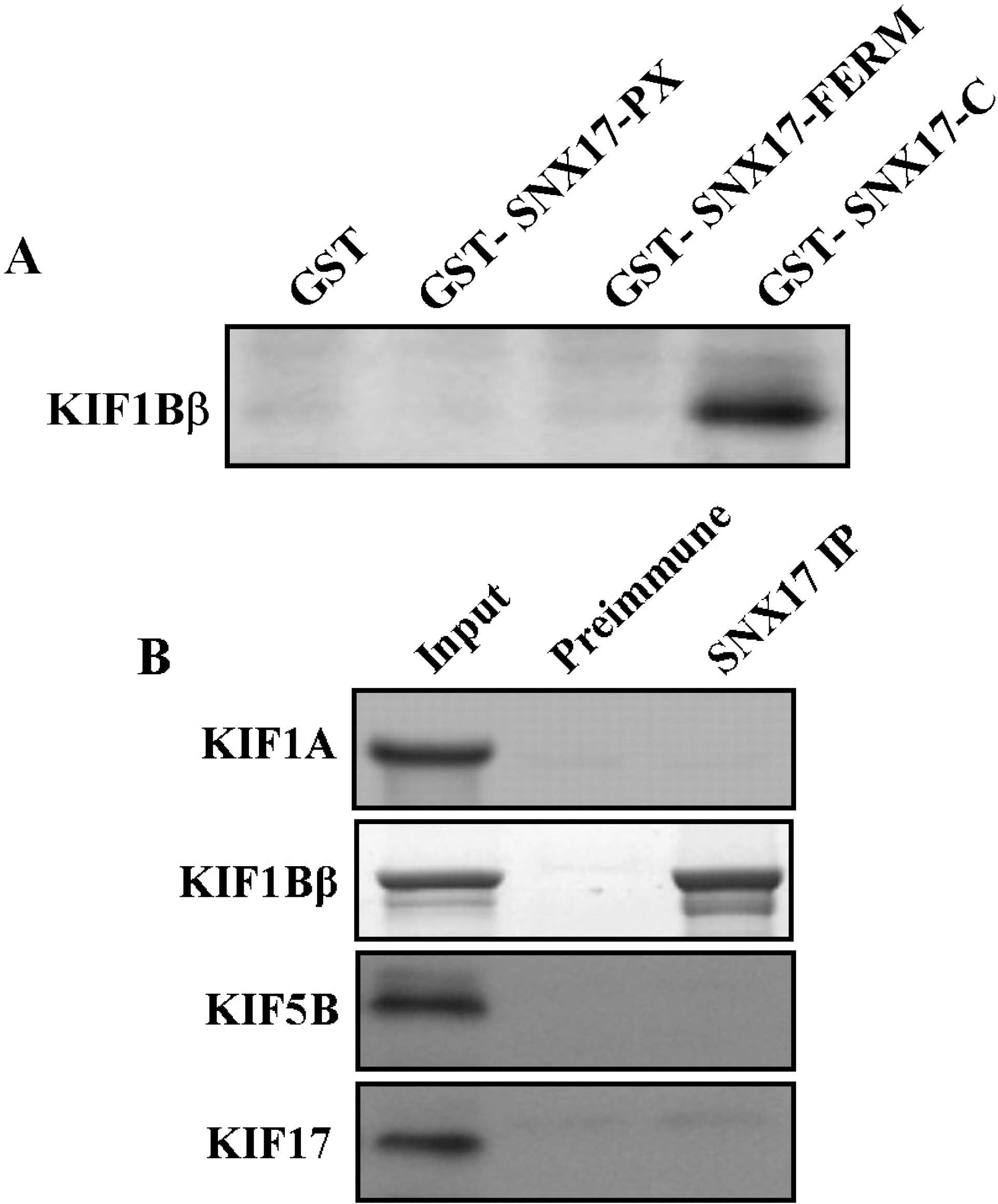
- KIF1B beta is a member of the Kinesin superfamily proteins (KIFs), which are microtubule-dependent molecular motors that are involved in various intracellular organellar transport processes. KIF1B beta is not restricted to neuronal systems, however, is widely expressed in other tissues, even though the function of KIF1B beta is still unclear. To elucidate the KIF1B beta-binding proteins in non-neuronal cells, we used the yeast two-hybrid system, and found a specific interaction of KIF1B beta and the sorting nexin (SNX) 17. The C-terminal region of SNX17 is required for the binding with KIF1B beta. SNX17 protein bound to the specific region of KIF1B beta (813-916. aa), but not to other kinesin family members. In addition, this specific interaction was also observed in the Glutathione S-transferase pull-down assay. An antibody to SNX17 specifically co-immunoprecipitated KIF1B beta associated with SNX17 from mouse brain extracts. These results suggest that SNX17 might be involved in the KIF1B beta-mediated transport as a KIF1B beta adaptor protein.
Keyword
MeSH Terms
Figure
Reference
-
Aizawa H., Sekine Y., Takemura R., Zhang Z., Nangaku M., Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 119:1287–1296. 1992.
ArticleBunn RC., Jensen MA., Reed BC. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 10:819–832. 1999.
ArticleBurden JJ., Sun XM., Garcia AB., Soutar AK. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem. 279:16237–16245. 2004.
ArticleChishti AH., Kim AC., Marfatia SM., Lutchman M., Hanspal M., Jindal H., Liu SC., Low PS., Rouleau GA., Mohandas N., Chasis JA., Conboy JG., Gascard P., Takakuwa Y., Huang SC., Benz EJ Jr., Bretscher A., Fehon RG., Gusella JF., Ramesh V., Solomon F., Marchesi VT., Tsukita S., Tsukita S., Arpin M., Louvard D., Tonks NK., Anderson JM., Fanning AS., Bryant PJ., Woods DF., Hoover KB. The FERM domain: aunique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 23:281–282. 1998.Dorner C., Ullrich A., Haring HU., Lammers R. The kinesin-like motor protein KIF1C occurs in intact cells as a dimer and associates with proteins of the 14-3-3 family. J Biol Chem. 274:33654–33660. 1999.
ArticleFlorian V., Schluter T., Bohnensack R. A new member of the sorting nexin family interacts with the C-terminus of P-selectin. Biochem Biophys Res Commun. 281:1045–1050. 2001.
ArticleGoldstein LS. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc Natl Acad Sci U S A. 98:6999–7003. 2001.
ArticleHirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 279:519–526. 1998.
ArticleKanai Y., Okada Y., Tanaka Y., Harada A., Terada S., Hirokawa N. KIF5C, A novel neuronal kinesin enriched in motor neurons. J Neurosci. 20:6374–6384. 2000.
ArticleKarcher RL., Deacon SW., Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12:21–27. 2002.
ArticleKim SJ., Lee CH., Park HY., Yea SS., Jang WH., Lee SK., Park YH., Jung YW., Seog DH. Kinesin superfamily KIF1B alpha protein binds to the PDZ domain of MALS-3. The Korean Journal of Anatomy. 39:375–382. 2006.Knauth P., Schluter T., Czubayko M., Kirsch C., Florian V., Schreckenberger S., Hahn H., Bohnensack R. Functions of sorting nexin 17 domains and recognition motif for P-selectin trafficking. J Mol Biol. 347:813–825. 2005.
ArticleKondo S., Sato-Yoshitake R., Noda Y., Aizawa H., Nakata T., Matsuura Y., Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 125:1095–1107. 1994.
ArticleMiki H., Setou M., Kaneshiro K Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 98:7004–7011. 2001.
ArticleMok H., Shin H., Kim S., Lee JR., Yoon J., Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 22:5253–5258. 2002.Nakagawa T., Setou M., Seog D., Ogasawara K., Dohmae N., Takio K., Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 103:569–581. 2000.
ArticleNangaku M., Sato-Yoshitake R., Okada Y., Noda Y., Takemura R., Yamazaki H., Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 79:1209–1220. 1994.
ArticleOkada Y., Higuchi H., Hirokawa N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature. 424:574–577. 2003.
ArticleOkada Y., Hirokawa N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science. 283:1152–1157. 1999.
ArticleOkada Y., Yamazaki H., Sekine-Aizawa Y., Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 81:769–780. 1995.Paik JE., Kim N., Yea AA., Jang WH., Chung JY., Lee SK., Park YH., Han J., Seog DH. Kinesin superfamily KIF5 proteins bind to (III spectrin. Korean J Physiol Pharmacol. 8:167–172. 2004.Sambrook J., Fritsch EF., Maniatis T. Molecular cloning: a laboratory manual. 3rd ed.Cold Spring Harbor Laboratory;Cold Spring Harbor, New York: 1989.Seog DH., Lee DH., Lee SK. Molecular Motor Proteins of the Kinesin superfamily proteins (KIFs): structure, cargo and disease. J Korean Medical Science. 19:1–7. 2004.
ArticleSetou M., Nakagawa T., Seog DH., Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 288:1796–1802. 2000.
ArticleSetou M., Seog DH. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 417:83–87. 2002.
ArticleSu Q., Cai Q., Gerwin C., Smith CL., Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol. 6:941–953. 2004.
ArticleTakeda S., Yamazaki H., Seog DH., Kanai Y., Terada S., Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 148:1255–1265. 2000.
Articlevan Kerkhof P., Lee J., McCormick L., Tetrault E., Lu W., Schoenfish M., Oorschot V., Strous GJ., Klumperman J., Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 24:2851–2861. 2005.
ArticleWang Y., Zhou Y., Szabo K., Haft CR., Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 13:1965–1976. 2002.
ArticleWilliams R., Schluter T., Roberts MS., Knauth P., Bohnensack R., Cutler DF. Sorting nexin 17 accelerates internalization yet retards degradation of P-selectin. Mol Biol Cell. 15:3095–3105. 2004.
ArticleWorby CA., Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 3:919–931. 2002.
ArticleYonekawa Y., Harada A., Okada Y., Funakoshi T., Kanai Y., Takei Y., Terada S., Noda T., Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 141:431–441. 1998.
ArticleZhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang HW., Terada S., Nakata T., Takei Y., Saito M., Tsuji S., Hayashi Y., Hirokawa N. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 105:587–597. 2001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Motor Proteins of the Kinesin Superfamily Proteins (KIFs): Structure, Cargo and Disease
- Kinesin Superfamily KIF1Balpha Protein Binds to the PDZ Domain of MALS-3
- Kinesin Spindle Protein Inhibition in Translational Research
- Kinesin Superfamily KIF1A Protein Binds to Synaptotagmin XI
- Kinesin Superfamily KIF5 Proteins Bind to betaIII Spectrin