Yonsei Med J.
2014 Sep;55(5):1196-1205. 10.3349/ymj.2014.55.5.1196.
LRIG1 Enhances Chemosensitivity by Modulating BCL-2 Expression and Receptor Tyrosine Kinase Signaling in Glioma Cells
- Affiliations
-
- 1Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China. chenqx666@sohu.com
- KMID: 1799481
- DOI: http://doi.org/10.3349/ymj.2014.55.5.1196
Abstract
- PURPOSE
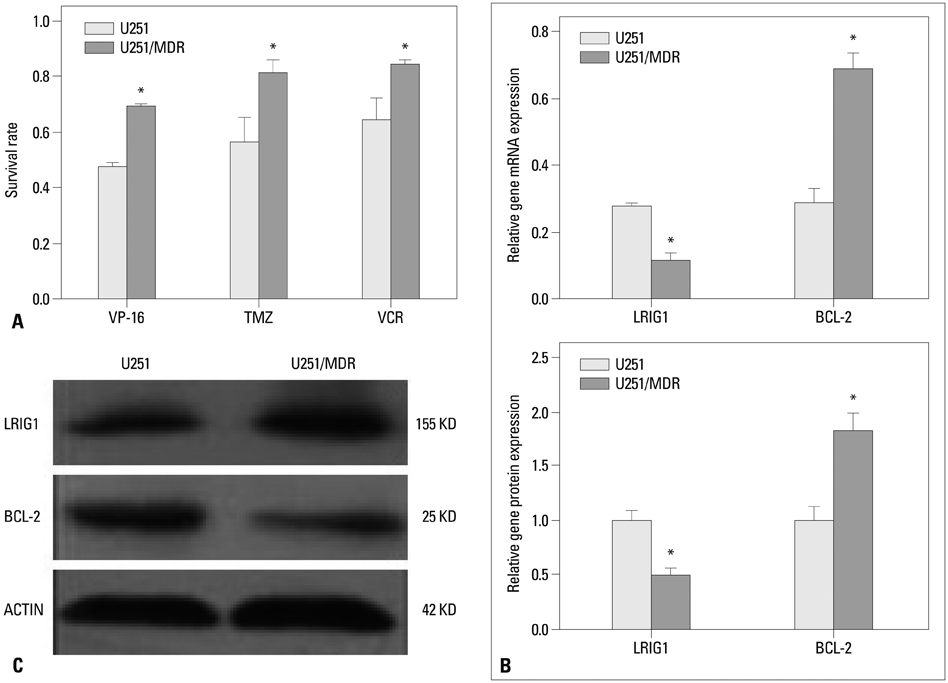
Leucine-rich repeats and immunoglobulin-like domains 1 (LRIG1) are an inhibitor of receptor tyrosine kinases (RTKs) that was discovered in recent years, and many studies showed that LRIG1 is a tumor suppressor gene and may be related to tumor drug resistance. In this study, we explored whether LRIG1 protein expression can improve the chemosensitivity of glioma cells and what was its mechanism.
MATERIALS AND METHODS
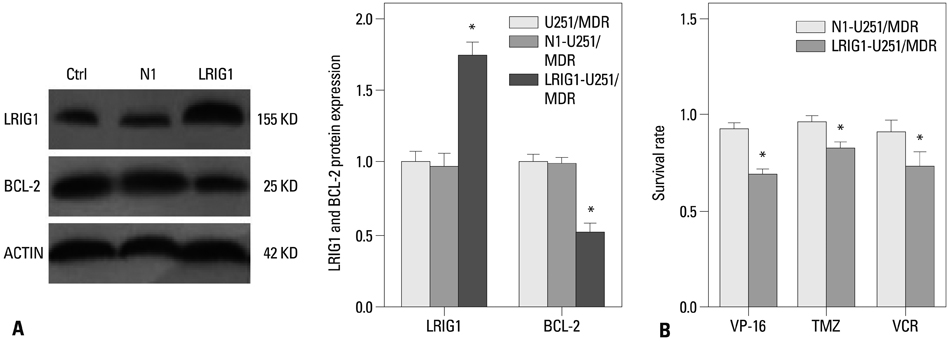
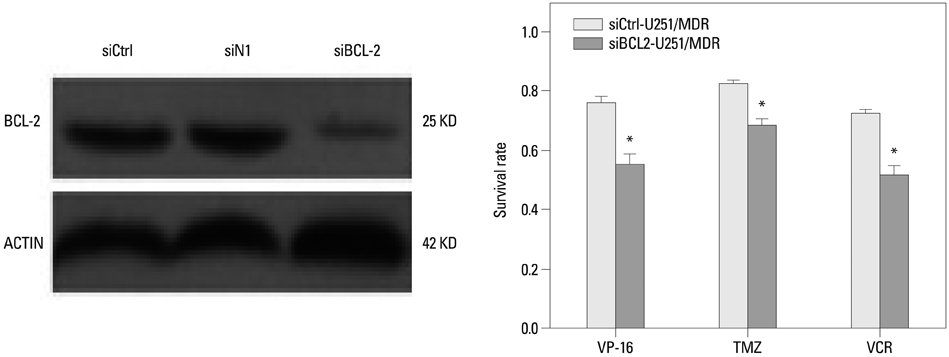
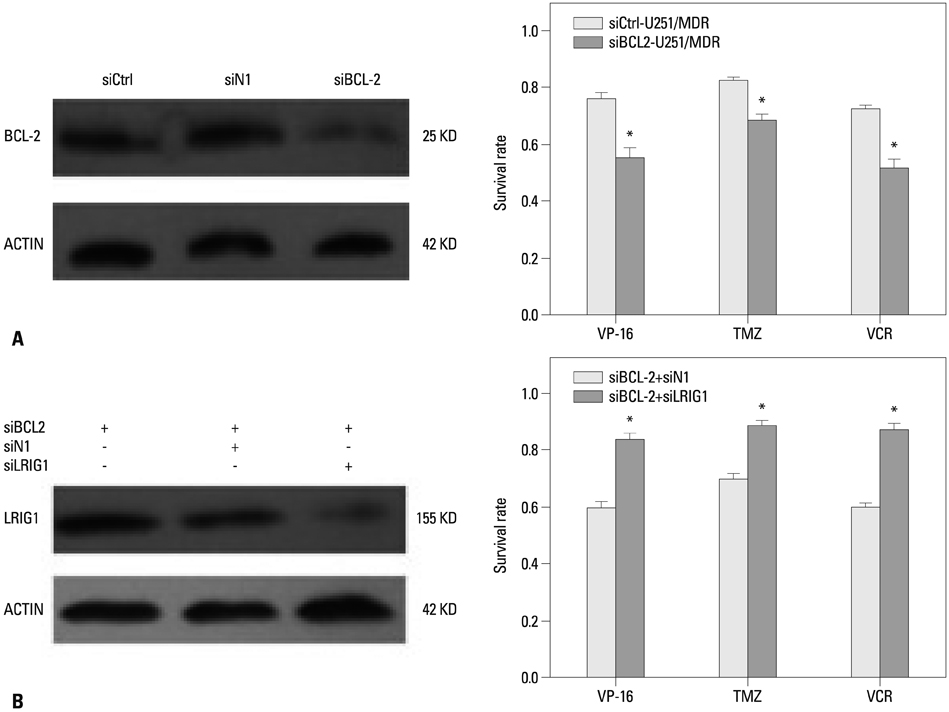
We collected 93 cases of glioma tissues and detected the expression of LRIG1 and BCL-2. We constructed a multidrug resistance cell line U251/multidrug resistance (MDR) and examined the change of LRIG1 and BCL-2 at mRNA and protein expression levels. LRIG1 expression was upregulated in U251/MDR cells and we detected the change of multidrug resistance. Meanwhile, we changed the expression of LRIG1 and BCL-2 and explored the relationship between LRIG1 and BCL-2. Finally, we also explored the relationship between LRIG1 and RTKs.
RESULTS
LRIG1 was negatively correlated with BCL-2 expression in glioma tissue and U251/MDR cells, and upregulation of LRIG1 can enhance chemosensitivity and inhibit BCL-2 expression. Furthermore, LRIG1 was negatively correlated with RTKs in U251/MDR cells.
CONCLUSION
These results demonstrated that LRIG1 can improve chemosensitivity by modulating BCL-2 expression and RTK signaling in glioma cells.
Keyword
MeSH Terms
-
Astrocytoma/drug therapy/genetics/metabolism
Cell Line, Tumor
Drug Resistance, Neoplasm/genetics/*physiology
Gene Expression Regulation, Neoplastic
Gene Knockdown Techniques
Glioma/drug therapy/*metabolism
Humans
Membrane Glycoproteins/metabolism/*physiology
Proto-Oncogene Proteins c-bcl-2/*metabolism
RNA, Messenger/metabolism
Receptor Protein-Tyrosine Kinases/metabolism
Membrane Glycoproteins
Proto-Oncogene Proteins c-bcl-2
RNA, Messenger
Receptor Protein-Tyrosine Kinases
Figure
Reference
-
1. Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011; 12:495–508.
Article2. Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001; 61:7501–7506.3. Hösli P, Sappino AP, de Tribolet N, Dietrich PY. Malignant glioma: should chemotherapy be overthrown by experimental treatments? Ann Oncol. 1998; 9:589–600.
Article4. Athanassiou H, Synodinou M, Maragoudakis E, Paraskevaidis M, Verigos C, Misailidou D, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005; 23:2372–2377.
Article5. Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007; 446:749–757.
Article6. Calatozzolo C, Gelati M, Ciusani E, Sciacca FL, Pollo B, Cajola L, et al. Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma. J Neurooncol. 2005; 74:113–121.
Article7. Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007; 25:4127–4136.
Article8. Jennings MT, Iyengar S. The molecular genetics of therapeutic resistance in malignant astrocytomas. Am J Pharmacogenomics. 2001; 1:93–99.
Article9. Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of temozolomide. Curr Med Chem. 2009; 16:245–257.
Article10. Suzuki Y, Sato N, Tohyama M, Wanaka A, Takagi T. cDNA cloning of a novel membrane glycoprotein that is expressed specifically in glial cells in the mouse brain. LIG-1, a protein with leucine-rich repeats and immunoglobulin-like domains. J Biol Chem. 1996; 271:22522–22527.
Article11. Nilsson J, Vallbo C, Guo D, Golovleva I, Hallberg B, Henriksson R, et al. Cloning, characterization, and expression of human LIG1. Biochem Biophys Res Commun. 2001; 284:1155–1161.
Article12. Nilsson J, Starefeldt A, Henriksson R, Hedman H. LRIG1 protein in human cells and tissues. Cell Tissue Res. 2003; 312:65–71.
Article13. Thomasson M, Hedman H, Guo D, Ljungberg B, Henriksson R. LRIG1 and epidermal growth factor receptor in renal cell carcinoma: a quantitative RT-PCR and immunohistochemical analysis. Br J Cancer. 2003; 89:1285–1289.
Article14. Ye F, Guo DS, Niu HQ, Tao SZ, Ou YB, Lu YP, et al. [Molecular mechanism of LRIG1 cDNA-induced apoptosis in human glioma cell line H4]. Ai Zheng. 2004; 23:1149–1154.15. Miller JK, Shattuck DL, Ingalla EQ, Yen L, Borowsky AD, Young LJ, et al. Suppression of the negative regulator LRIG1 contributes to ErbB2 overexpression in breast cancer. Cancer Res. 2008; 68:8286–8294.
Article16. Thomasson M, Wang B, Hammarsten P, Dahlman A, Persson JL, Josefsson A, et al. LRIG1 and the liar paradox in prostate cancer: a study of the expression and clinical significance of LRIG1 in prostate cancer. Int J Cancer. 2011; 128:2843–2852.
Article17. Lu L, Teixeira VH, Yuan Z, Graham TA, Endesfelder D, Kolluri K, et al. LRIG1 regulates cadherin-dependent contact inhibition directing epithelial homeostasis and pre-invasive squamous cell carcinoma development. J Pathol. 2013; 229:608–620.
Article18. Shattuck DL, Miller JK, Laederich M, Funes M, Petersen H, Carraway KL 3rd, et al. LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol. 2007; 27:1934–1946.
Article19. Sheu JJ, Lee CC, Hua CH, Li CI, Lai MT, Lee SC, et al. LRIG1 modulates aggressiveness of head and neck cancers by regulating EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling. Oncogene. 2014; 33:1375–1384.
Article20. Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012; 149:146–158.
Article21. Yi W, Haapasalo H, Holmlund C, Järvelä S, Raheem O, Bergenheim AT, et al. Expression of leucine-rich repeats and immunoglobulin-like domains (LRIG) proteins in human ependymoma relates to tumor location, WHO grade, and patient age. Clin Neuropathol. 2009; 28:21–27.
Article22. Jansen B, Schlagbauer-Wadl H, Brown BD, Bryan RN, van Elsas A, Müller M, et al. bcl-2 antisense therapy chemosensitizes human melanoma in SCID mice. Nat Med. 1998; 4:232–234.
Article23. Le Calvé B, Rynkowski M, Le Mercier M, Bruyère C, Lonez C, Gras T, et al. Long-term in vitro treatment of human glioblastoma cells with temozolomide increases resistance in vivo through up-regulation of GLUT transporter and aldo-keto reductase enzyme AKR1C expression. Neoplasia. 2010; 12:727–739.
Article24. Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005; 103:89–101.
Article25. Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, et al. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009; 2:ra4.
Article26. Martens T, Schmidt NO, Eckerich C, Fillbrandt R, Merchant M, Schwall R, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006; 12(20 Pt 1):6144–6152.
Article27. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008; 455:1061–1068.28. Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007; 318:287–290.
Article29. Hedman H, Nilsson J, Guo D, Henriksson R. Is LRIG1 a tumour suppressor gene at chromosome 3p14.3? Acta Oncol. 2002; 41:352–354.
Article30. Goldoni S, Iozzo RA, Kay P, Campbell S, McQuillan A, Agnew C, et al. A soluble ectodomain of LRIG1 inhibits cancer cell growth by attenuating basal and ligand-dependent EGFR activity. Oncogene. 2007; 26:368–381.
Article31. Hansford LM, Marshall GM. Glial cell line-derived neurotrophic factor (GDNF) family ligands reduce the sensitivity of neuroblastoma cells to pharmacologically induced cell death, growth arrest and differentiation. Neurosci Lett. 2005; 389:77–82.
Article32. Ledda F, Bieraugel O, Fard SS, Vilar M, Paratcha G. Lrig1 is an endogenous inhibitor of Ret receptor tyrosine kinase activation, downstream signaling, and biological responses to GDNF. J Neurosci. 2008; 28:39–49.
Article33. Li F, Yang W, Guo D, Hu Z, Xu H, Ye Z. LRIG1 combined with cisplatin enhances bladder cancer lesions via a novel pathway. Oncol Rep. 2011; 25:1629–1637.
Article34. Liu B, Chen Q, Tian D, Wu L, Wang J, Cai Q, et al. Increased leucine-rich repeats and immunoglobulin-like domains 1 expression enhances chemosensitivity in glioma. Neural Regen Res. 2011; 32:2516–2520.35. Wesarg E, Hoffarth S, Wiewrodt R, Kröll M, Biesterfeld S, Huber C, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007; 121:2387–2394.
Article36. He X, Lin B, Kong L, Zhang J. The potential mechanism of chemosensitive difference between 2 types of ovarian cancer. Saudi Med J. 2007; 28:1044–1049.37. Nordfors K, Haapasalo J, Helén P, Paetau A, Paljärvi L, Kalimo H, et al. Peroxiredoxins and antioxidant enzymes in pilocytic astrocytomas. Clin Neuropathol. 2007; 26:210–218.
Article38. Sekine I, Shimizu C, Nishio K, Saijo N, Tamura T. A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with breast cancer. Int J Clin Oncol. 2009; 14:112–119.
Article39. Wang P, Zhen H, Jiang X, Zhang W, Cheng X, Guo G, et al. Boron neutron capture therapy induces apoptosis of glioma cells through Bcl-2/Bax. BMC Cancer. 2010; 10:661.
Article40. Gur G, Rubin C, Katz M, Amit I, Citri A, Nilsson J, et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004; 23:3270–3281.
Article41. Ye F, Gao Q, Xu T, Zeng L, Ou Y, Mao F, et al. Upregulation of LRIG1 suppresses malignant glioma cell growth by attenuating EGFR activity. J Neurooncol. 2009; 94:183–194.
Article42. Khatua S, Peterson KM, Brown KM, Lawlor C, Santi MR, LaFleur B, et al. Overexpression of the EGFR/FKBP12/HIF-2alpha pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003; 63:1865–1870.43. Lei W, Mayotte JE, Levitt ML. Enhancement of chemosensitivity and programmed cell death by tyrosine kinase inhibitors correlates with EGFR expression in non-small cell lung cancer cells. Anticancer Res. 1999; 19:221–228.44. Nogi H, Kobayashi T, Suzuki M, Tabei I, Kawase K, Toriumi Y, et al. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol Rep. 2009; 21:413–417.
Article45. Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, et al. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010; 70:5184–5193.
Article46. Li Y, Guessous F, DiPierro C, Zhang Y, Mudrick T, Fuller L, et al. Interactions between PTEN and the c-Met pathway in glioblastoma and implications for therapy. Mol Cancer Ther. 2009; 8:376–385.
Article47. Lin CI, Whang EE, Donner DB, Du J, Lorch J, He F, et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010; 8:1217–1226.
Article48. Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007; 67:4408–4417.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "LRIG1 Enhances Chemosensitivity by Modulating BCL-2 Expression and Receptor Tyrosine Kinase Signaling in Glioma Cells" by Guo Z, et al. (Yonsei Med J 2014;55:1196-205.)
- Identification of SAP as a CTLA-4 Binding Molecule: a Role of SAP in CTLA-4 Signaling Proposed
- Tyrosine Protein Kinase (trk) Receptors Expression in Malignant Melanoma Cells
- The Activation of ERK1/2 Via Tyrosine Kinase Pathway Attenuates TRAIL-induced Apoptosis in HeLa cell
- Activation of DDR2 Involved in Atherosclerosis by Oxidative Stress