J Korean Med Sci.
2014 Jan;29(1):90-97. 10.3346/jkms.2014.29.1.90.
Comparison of Acarbose and Voglibose in Diabetes Patients Who Are Inadequately Controlled with Basal Insulin Treatment: Randomized, Parallel, Open-Label, Active-Controlled Study
- Affiliations
-
- 1Division of Endocrinology & Metabolism, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Division of Endocrinology & Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. cdongs@kumc.or.kr
- 3Division of Endocrinology & Metabolism, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Endocrinology & Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 5Division of Endocrinology & Metabolism, Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 6Division of Endocrinology & Metabolism, Department of Internal Medicine, Hallym University College of Medicine, Seoul, Korea.
- 7Division of Endocrinology & Metabolism, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 8Division of Endocrinology & Metabolism, Department of Internal Medicine, Pusan National University College of Medicine, Busan, Korea.
- 9Division of Endocrinology & Metabolism, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 10Division of Endocrinology & Metabolism, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 11Division of Endocrinology & Metabolism, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea.
- 12Bayer Korea Ltd., Seoul, Korea.
- KMID: 1796920
- DOI: http://doi.org/10.3346/jkms.2014.29.1.90
Abstract
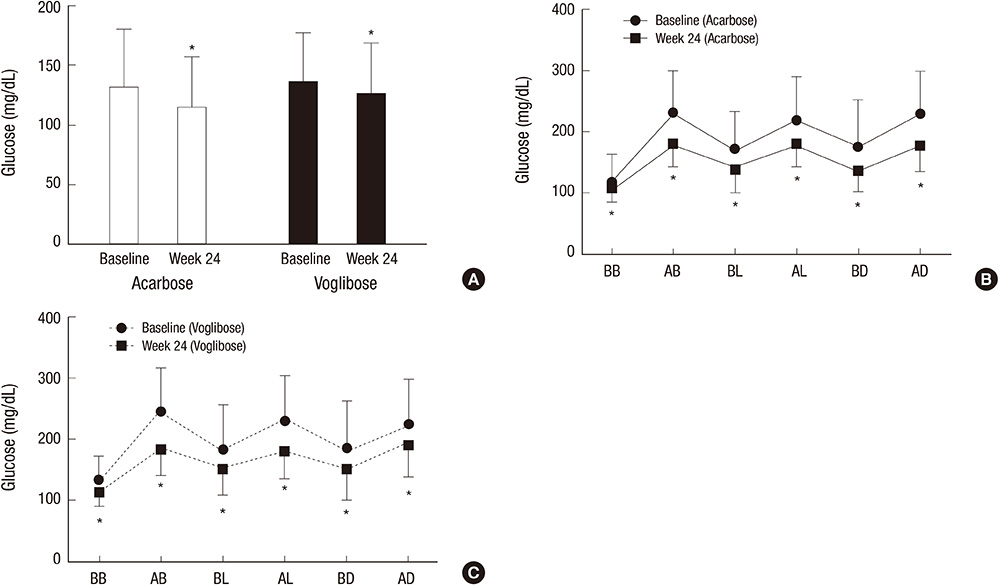
- We studied the efficacy and safety of acarbose in comparison with voglibose in type 2 diabetes patients whose blood glucose levels were inadequately controlled with basal insulin alone or in combination with metformin (or a sulfonylurea). This study was a 24-week prospective, open-label, randomized, active-controlled multi-center study. Participants were randomized to receive either acarbose (n=59, 300 mg/day) or voglibose (n=62, 0.9 mg/day). The mean HbA1c at week 24 was significantly decreased approximately 0.7% from baseline in both acarbose (from 8.43% +/- 0.71% to 7.71% +/- 0.93%) and voglibose groups (from 8.38% +/- 0.73% to 7.68% +/- 0.94%). The mean fasting plasma glucose level and self-monitoring of blood glucose data from 1 hr before and after each meal were significantly decreased at week 24 in comparison to baseline in both groups. The levels 1 hr after dinner at week 24 were significantly decreased in the acarbose group (from 233.54 +/- 69.38 to 176.80 +/- 46.63 mg/dL) compared with the voglibose group (from 224.18 +/- 70.07 to 193.01 +/- 55.39 mg/dL). In conclusion, both acarbose and voglibose are efficacious and safe in patients with type 2 diabetes who are inadequately controlled with basal insulin. (ClinicalTrials.gov number, NCT00970528)
Keyword
MeSH Terms
-
Acarbose/adverse effects/*therapeutic use
Blood Glucose
Diabetes Mellitus, Type 2/blood/*drug therapy
Enzyme Inhibitors/adverse effects/therapeutic use
Female
Hemoglobin A, Glycosylated/analysis
Humans
Hypoglycemic Agents/adverse effects/therapeutic use
Inositol/adverse effects/*analogs & derivatives/therapeutic use
Insulin/*blood/therapeutic use
Male
Metformin/therapeutic use
Middle Aged
Prospective Studies
alpha-Glucosidases/antagonists & inhibitors
Acarbose
Blood Glucose
Enzyme Inhibitors
Hemoglobin A, Glycosylated
Hypoglycemic Agents
Insulin
Inositol
Metformin
alpha-Glucosidases
Figure
Reference
-
1. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–2653.2. Bergenstal RM, Johnson M, Powers MA, Wynne A, Vlajnic A, Hollander P, Rendell M. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008; 31:1305–1310.3. Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK. 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009; 361:1736–1747.4. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012; 55:1577–1596.5. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–1379.6. Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008; 371:1073–1084.7. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007; 30:263–269.8. Abrahamson MJ, Peters A. Intensification of insulin therapy in patients with type 2 diabetes mellitus: an algorithm for basal-bolus therapy. Ann Med. 2012; 44:836–846.9. Raccah D. Options for the intensification of insulin therapy when basal insulin is not enough in type 2 diabetes mellitus. Diabetes Obes Metab. 2008; 10:76–82.10. Coniff R, Krol A. Acarbose: a review of US clinical experience. Clin Ther. 1997; 9:16–26.11. Scheen AJ. Drug treatment of non-insulin-dependent diabetes mellitus in the 1990s: achievements and future developments. Drugs. 1997; 54:355–368.12. Fujisawa T, Ikegami H, Inoue K, Kawabata Y, Ogihara T. Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism. 2005; 54:387–390.13. Göke B, Fuder H, Wieckhorst G, Theiss U, Stridde E, Littke T, Kleist P, Arnold R, Lücker PW. Voglibose (AO-128) is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve. Digestion. 1995; 56:493–501.14. Vichayanrat A, Ploybutr S, Tunlakit M, Watanakejorn P. Efficacy and safety of voglibose in comparison with acarbose in type 2 diabetic patients. Diabetes Res Clin Pract. 2002; 55:99–103.15. Roze S, Valentine WJ, Evers T, Palmer AJ. Acarbose in addition to existing treatments in patients with type 2 diabetes: health economic analysis in a German setting. Curr Med Res Opin. 2006; 22:1415–1424.16. Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients: the Essen Study. Diabetes Care. 1994; 17:561–566.17. Internaltional Diabetes Federation. Global guideline for type 2 diabetes. accessed on 19 February 2013. Available at http://www.idf.org/global-guideline-type-2-diabetes-2012.18. Service FJ, Hall LD, Westland RE, O'Brien PC, Go VL, Haymond MW, Rizza RA. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. 1983; 25:316–321.19. Hwu CM, Ho LT, Fuh MM, Siu SC, Sutanegara D, Piliang S, Chan JC. Asian Acarbose Study Group. Acarbose improves glycemic control in insulin-treated Asian type 2 diabetic patients: results from a multinational, placebo-controlled study. Diabetes Res Clin Pract. 2003; 60:111–118.20. Kim MK, Suk JH, Kwon MJ, Chung HS, Yoon CS, Jun HJ, Ko JH, Kim TK, Lee SH, Oh MK, et al. Nateglinide and acarbose for postprandial glucose control after optimizing fasting glucose with insulin glargine in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011; 92:322–328.21. Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, McGill JB. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM: a placebo-controlled dose-comparison study. Diabetes Care. 1995; 18:817–824.22. Coniff RF, Shapiro JA, Seaton TB, Hoogwerf BJ, Hunt JA. A double-blind placebo-controlled trial evaluating the safety and efficacy of acarbose for the treatment of patients with insulin-requiring type II diabetes. Diabetes Care. 1995; 18:928–932.23. Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study. Am J Med. 1997; 103:483–490.24. Hanefeld M, Fischer S, Schulze J, Spengler M, Wargenau M, Schollberg K, Fücker K. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with diet alone. Diabetes Care. 1991; 14:732–737.25. Meneghini LF, Orozco-Beltran D, Khunti K, Caputo S, Damçi T, Liebl A, Ross SA. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011; 96:3337–3353.26. Gross JL, Kramer CK, Leitão CB, Hawkins N, Viana LV, Schaan BD, Pinto LC, Rodrigues TC, Azevedo MJ. Diabetes and Endocrinology Meta-analysis Group (DEMA). Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med. 2011; 154:672–679.27. McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, Dahl M. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011; 5:e35–e48.28. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010; 303:1410–1418.29. Hegele RA, Connelly PW, Palmason C, Jenkins DJ, Wolever TM. Differential response of plasma lipoprotein(a) and apolipoprotein B in NIDDM subjects treated with acarbose. Diabetes Care. 1995; 18:272–273.30. Wolever TM, Radmard R, Chiasson JL, Hunt JA, Josse RG, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH. One-year acarbose treatment raises fasting serum acetate in diabetic patients. Diabet Med. 1995; 12:164–172.31. Jenkins DJ, Wolever TM, Jenkins A, Brighenti F, Vuksan V, Rao AV, Cunnane SC, Ocana A, Corey P, Vezina C, et al. Specific types of colonic fermentation may raise low-density-lipoprotein-cholesterol concentrations. Am J Clin Nutr. 1991; 54:141–147.32. Zeymer U. Cardiovascular benefits of acarbose in impaired glucose tolerance and type 2 diabetes. Int J Cardiol. 2006; 107:11–20.33. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003; 290:486–494.34. Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol. 2007; 6:20.35. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002; 359:2072–2077.36. Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, Renard E, Russell-Jones D, Philotheou A, Francisco AM, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012; 379:1489–1497.37. Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008; 51:408–416.38. Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001; 24:631–636.39. Su JB, Wang XQ, Chen JF, Wu G, Jin Y. Glycemic variability in insulin treated type 2 diabetes with well-controlled hemoglobin A1c and its response to further treatment with acarbose. Chin Med J (Engl). 2011; 124:144–147.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Study about the Effects of Acarbose and Voglibose in Type 2 Diabetic Patients
- The Effect of Acarbose as an Adjuvant Therapy in Sulfonylurea-Treated NIDDM Patients
- Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial
- Exenatide versus Insulin Lispro Added to Basal Insulin in a Subgroup of Korean Patients with Type 2 Diabetes Mellitus
- Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study