J Korean Med Sci.
2013 Mar;28(3):366-373. 10.3346/jkms.2013.28.3.366.
Overexpression of Human Arginine Decarboxylase Rescues Human Mesenchymal Stem Cells against H2O2 Toxicity through Cell Survival Protein Activation
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1786932
- DOI: http://doi.org/10.3346/jkms.2013.28.3.366
Abstract
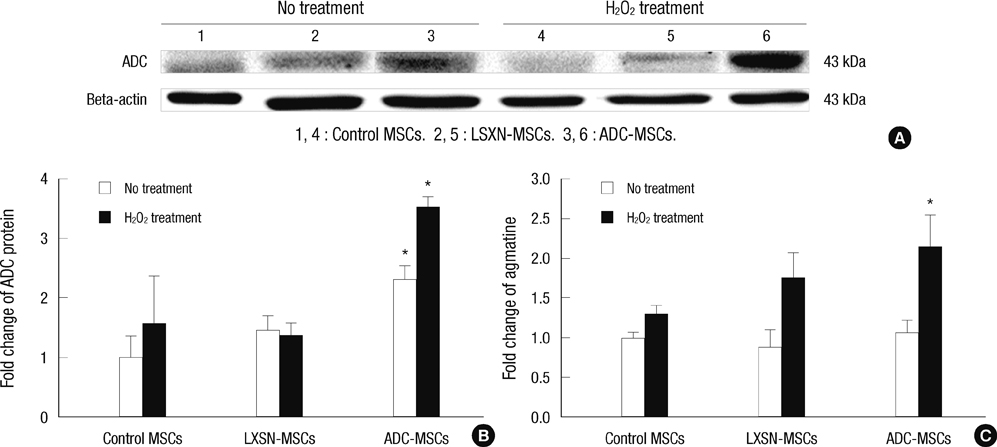
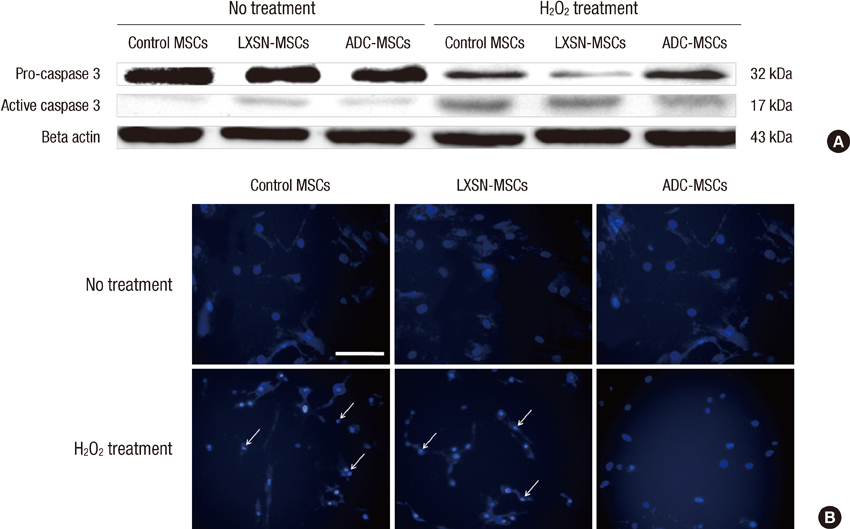
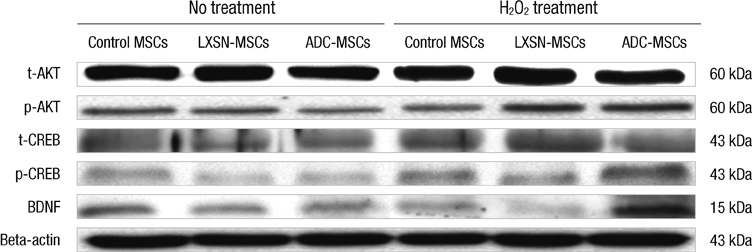
- In this study, we explored the potentiality of human arginine decarboxylase (ADC) to enhance the survival of mesenchymal stem cells (MSCs) against unfavorable milieu of host tissues as the low survival of MSCs is the issue in cell transplantation therapy. To address this, human MSCs overexpressing human ADC were treated with H2O2 and the resultant intracellular events were examined. First, we examined whether human ADC is overexpressed in human MSCs. Then, we investigated cell survival or death related events. We found that the overexpression of human ADC increases formazan production and reduces caspase 3 activation and the numbers of FITC, hoechst, or propidium iodide positive cells in human MSCs exposed to H2O2. To elucidate the factors underlying these phenomena, AKT, CREB, and BDNF were examined. We found that the overexpression of human ADC phosphorylates AKT and CREB and increases BDNF level in human MSCs exposed to H2O2. The changes of these proteins are possibly relevant to the elevation of agmatine. Collectively, our data demonstrate that the overexpression of human ADC stimulates pro-survival factors to protect human MSCs against H2O2 toxicity. In conclusion, the present findings support that ADC can enhance the survival of MSCs against hostile environment of host tissues.
Keyword
MeSH Terms
-
Apoptosis/*drug effects
Brain-Derived Neurotrophic Factor/metabolism
Carboxy-Lyases/genetics/*metabolism
Caspase 3/metabolism
Cells, Cultured
Cyclic AMP Response Element-Binding Protein/metabolism
Humans
Hydrogen Peroxide/*toxicity
Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/cytology/drug effects/metabolism
Phosphorylation
Proto-Oncogene Proteins c-akt/metabolism
Brain-Derived Neurotrophic Factor
Cyclic AMP Response Element-Binding Protein
Hydrogen Peroxide
Proto-Oncogene Proteins c-akt
Caspase 3
Carboxy-Lyases
Figure
Cited by 1 articles
-
Agmatine Improves Cognitive Dysfunction and Prevents Cell Death in a Streptozotocin-Induced Alzheimer Rat Model
Juhyun Song, Bo Eun Hur, Kiran Kumar Bokara, Wonsuk Yang, Hyun Jin Cho, Kyung Ah Park, Won Taek Lee, Kyoung Min Lee, Jong Eun Lee
Yonsei Med J. 2014;55(3):689-699. doi: 10.3349/ymj.2014.55.3.689.
Reference
-
1. Morris SM Jr. Enzymes of arginine metabolism. J Nutr. 2004. 134:2743S–2747S.2. Cunin R, Glansdorff N, Piérard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986. 50:314–352.3. Borrell A, Culianez-Macia FA, Altabella T, Besford RT, Flores D, Tiburcio AF. Arginine decarboxylase is localized in chloroplasts. Plant Physiol. 1995. 109:771–776.4. Morrissey J, McCracken R, Ishidoya S, Klahr S. Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int. 1995. 47:1458–1461.5. Kasinathan V, Wingler A. Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Physiol Plant. 2004. 121:101–107.6. Alvarez-Ordóñez A, Fernández A, Bernardo A, López M. Arginine and lysine decarboxylases and the acid tolerance response of Salmonella Typhimurium. Int J Food Microbiol. 2010. 136:278–282.7. Wolf C, Brüss M, Hänisch B, Göthert M, von Kügelgen I, Molderings GJ. Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol. 2007. 71:276–283.8. Zhu MY, Wang WP, Huang J, Regunathan S. Chronic treatment with glucocorticoids alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. J Neurochem. 2007. 103:1811–1820.9. Zhu MY, Wang WP, Huang J, Feng YZ, Regunathan S, Bissette G. Repeated immobilization stress alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. Neurochem Int. 2008. 53:346–354.10. Regunathan S, Reis DJ. Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J Neurochem. 2000. 74:2201–2208.11. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010. 12:87–117.12. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005. 2:8.13. Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation. 2003. 107:929–934.14. Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010. 21:1513–1526.15. Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006. 24:416–425.16. Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006. 7:101–108.17. Ko E, Lee KY, Hwang DS. Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells Dev. 2012. 21:1877–1886.18. Chen HY, Zhang X, Chen SF, Zhang YX, Liu YH, Ma LL, Wang LX. The protective effect of 17β-estradiol against hydrogen peroxide-induced apoptosis on mesenchymal stem cell. Biomed Pharmacother. 2012. 66:57–63.19. Li S, Bian H, Liu Z, Wang Y, Dai J, He W, Liao X, Liu R, Luo J. Chlorogenic acid protects MSCs against oxidative stress by altering FOXO family genes and activating intrinsic pathway. Eur J Pharmacol. 2012. 674:65–72.20. Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H, Schupp N, Kassem M, Jakob F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006. 24:1226–1235.21. Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009. 46:241–281.22. Satoh T, Sakai N, Enokido Y, Uchiyama Y, Hatanaka H. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J Biochem. 1996. 120:540–546.23. Moon SU, Kwon KH, Kim JH, Bokara KK, Park KA, Lee WT, Lee JE. Recombinant hexahistidine arginine decarboxylase (hisADC) induced endogenous agmatine synthesis during stress. Mol Cell Biochem. 2010. 345:53–60.24. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986. 89:271–277.25. Müller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006. 41:876–884.26. Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, Müller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010. 114:e107–e116.27. Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM, Roudkenar MH. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012. 17:553–565.28. Li Z, Wei H, Liu X, Hu S, Cong X, Chen X. LPA rescues ER stress-associated apoptosis in hypoxia and serum deprivation-stimulated mesenchymal stem cells. J Cell Biochem. 2010. 111:811–820.29. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. 1995. New York: John Wiley & Sons.30. Coffin JM, Hughes SH, Varmus HE. Retroviruses. 1997. Plainview: Cold Spring Harbor Laboratory Press.31. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999. 6:99–104.32. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007. 129:1261–1274.33. Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998. 273:32377–32379.34. Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999. 286:2358–2361.35. Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Brain-derived neurotrophic factor prevents phencyclidine-induced apoptosis in developing brain by parallel activation of both the ERK and PI-3K/Akt pathways. Neuropharmacology. 2010. 58:330–336.36. Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999. 100:2373–2379.37. Nagai-Kusuhara A, Nakamura M, Mukuno H, Kanamori A, Negi A, Seigel GM. cAMP-responsive element binding protein mediates a cGMP/protein kinase G-dependent anti-apoptotic signal induced by nitric oxide in retinal neuro-glial progenitor cells. Exp Eye Res. 2007. 84:152–162.38. Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. 2008. 270:70–76.39. Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002. 22:275–279.40. Santhanam AV, Viswanathan S, Dikshit M. Activation of protein kinase B/Akt and endothelial nitric oxide synthase mediates agmatine-induced endothelium-dependent relaxation. Eur J Pharmacol. 2007. 572:189–196.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Generation of Insulin-Producing Human Mesenchymal Stem Cells Using Recombinant Adeno-Associated Virus
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- Recent Advances for Enhancing Drug Metabolizing Functions of Hepatocyte-like Cells Derived from Human Pluripotent Stem Cells
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients