Yonsei Med J.
2007 Feb;48(1):109-119. 10.3349/ymj.2007.48.1.109.
Generation of Insulin-Producing Human Mesenchymal Stem Cells Using Recombinant Adeno-Associated Virus
- Affiliations
-
- 1Department of Medical Engineering, Yonsei University College of Medicine, Seoul, Korea. hwal@yumc.yonsei.ac.kr
- 2National BK 21 Project Team of Nanobiomaterials for the Cell-based Implants, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1093531
- DOI: http://doi.org/10.3349/ymj.2007.48.1.109
Abstract
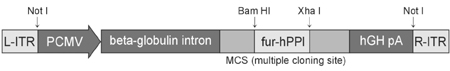
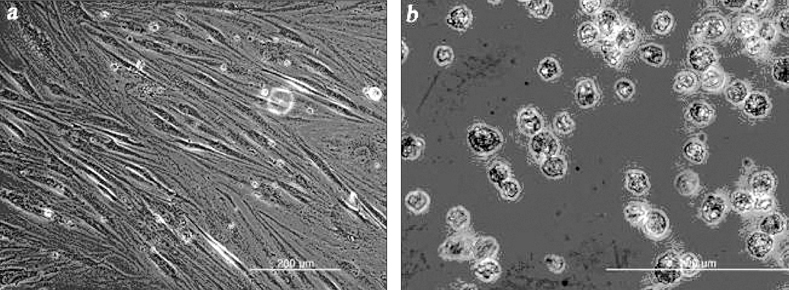
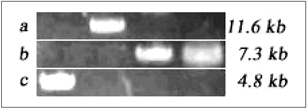
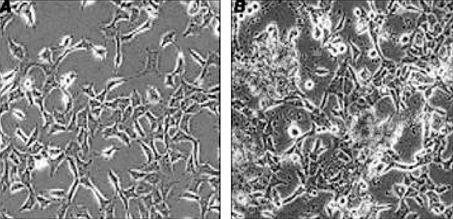
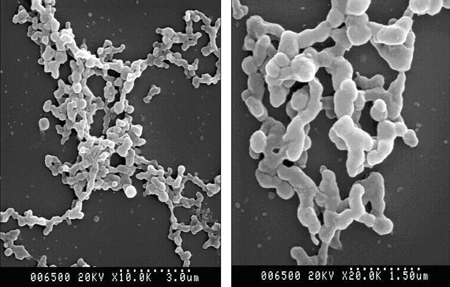
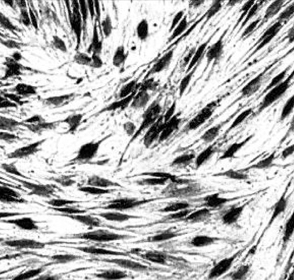
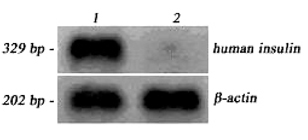
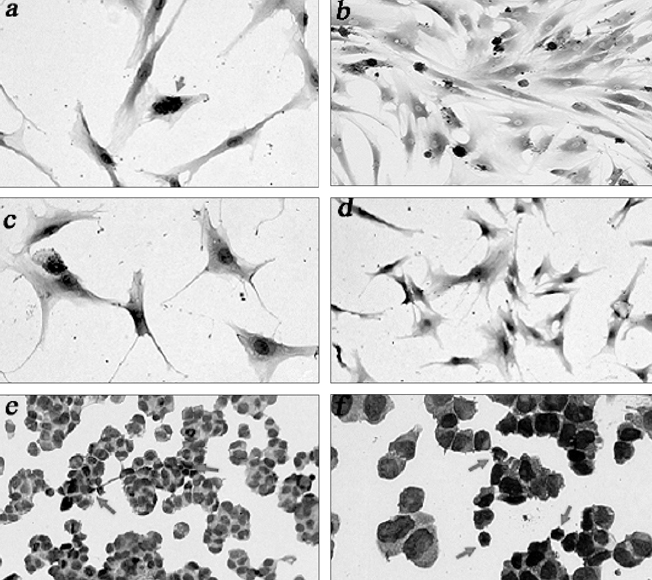
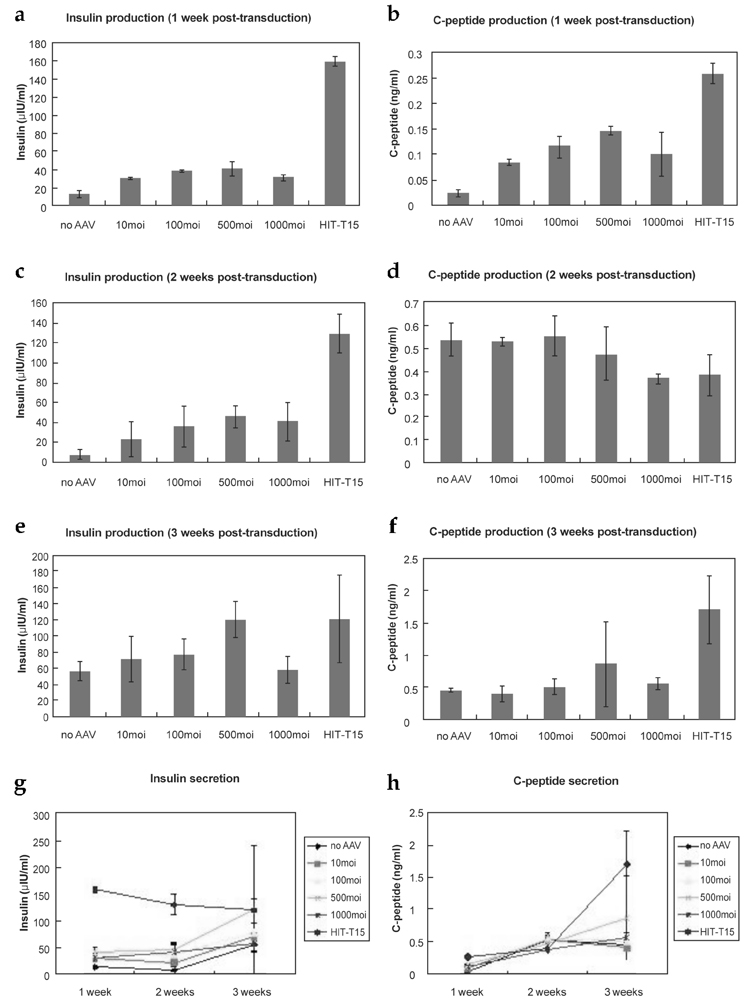
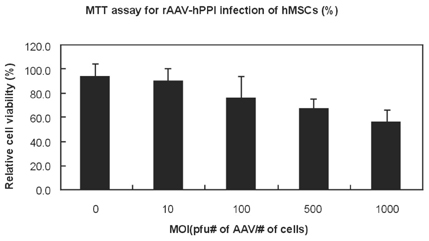
- The purpose of current experiment is the generation of insulin-producing human mesenchymal stem cells as therapeutic source for the cure of type 1 diabetes. Type 1 diabetes is generally caused by insulin deficiency accompanied by the destruction of islet beta-cells. In various trials for the treatment of type 1 diabetes, cell-based gene therapy using stem cells is considered as one of the most useful candidate for the treatment. In this experiment, human mesenchymal stem cells were transduced with AAV which is containing furin-cleavable human preproinsulin gene to generate insulin-producing cells as surrogate beta-cells for the type 1 diabetes therapy. In the rAAV production procedure, rAAV was generated by transfection of AD293 cells. Human mesenchymal stems cells were transduced using rAAV with a various multiplicity of infection. Transduction of recombinant AAV was also tested using beta-galactosidse expression. Cell viability was determined by using MTT assay to evaluate the toxicity of the transduction procedure. Expression and production of Insulin were tested using reverse transcriptase-polymerase chain reaction and immunocytochemistry. Secretion of human insulin and C-peptide from the cells was assayed using enzyme-linked immunosorbent assay. Production of insulin and C-peptide from the test group represented a higher increase compared to the control group. In this study, we examined generation of insulin-producing cells from mesenchymal stem cells by genetic engineering for diabetes therapy. This work might be valuable to the field of tissue engineering for diabetes treatment.
Figure
Cited by 1 articles
-
Retinoic Acid-induced Differentiation of Rat Mesenchymal Stem Cells into -Cell Lineage
Hyung Kim Jae, Sik Kim Kyung, Woo Lee Sang, Woo Kim Hyun, Jin Joo Dong, Seun Kim Yu, Hwal Suh
J Korean Soc Transplant. 2015;29(3):118-129. doi: 10.4285/jkstn.2015.29.3.118.
Reference
-
1. Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000. 408:483–488.2. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.3. Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003. 35:2140–2141.4. Snyder RO. Adeno-associated virus-mediated gene delivery. J Gene Med. 1999. 1:166–175.5. Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005. 16:541–550.6. Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterization of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004. 295:395–406.7. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999. 5:309–313.8. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogenic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002. 99:8932–8937.9. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.10. Pittenger MF, Marshak DR. Marshak DR, Gardner RL, Gottlieb D, editors. Mesenchymal stem cells of human adult bone marrow. Stem Cell Biology. 2001. New York: Cold Spring Harbor Laboratory Press;349–373.11. Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999. 47:499–506.12. Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989. 63:3822–3828.13. Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998. 72:2224–2232.14. Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther. 1998. 9:695–706.15. Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005. 23:594–604.16. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986. 89:271–277.17. Hay CW, Docherty K. Enhanced expression of a furin-cleavable proinsulin. J Mol Endocrinol. 2003. 31:597–607.18. Yang YW, Hsieh YC. Regulated secretion of proinsulin/insulin from human hepatoma cells transduced by recombinant adeno-associated virus. Biotechnol Appl Biochem. 2001. 33:133–140.19. Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994. 269:6241–6245.20. Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001. 12:117–129.21. Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S, et al. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003. 10:490–503.22. Srivastava A. Hematopoietic stem cell transduction by recombinant adeno-associated virus vectors: problems and solutions. Hum Gene Ther. 2005. 16:792–798.23. Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005. 102:17430–17435.24. Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999. 401:517–518.25. Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003. 348:255–256.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy
- Induction of Nestin Early Expression as a Hallmark for Mesenchymal Stem Cells Expression of PDX-1 as a Pre-disposing Factor for Their Conversion into Insulin Producing Cells
- Recombinant AAV Vector with MITF-M Promoter for Melanoma Gene Therapy
- Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease