J Bacteriol Virol.
2012 Mar;42(1):57-62. 10.4167/jbv.2012.42.1.57.
Recombinant AAV Vector with MITF-M Promoter for Melanoma Gene Therapy
- Affiliations
-
- 1Department of Agricultural Biology, National Academy of Agricultural Science, Rural Development Association, Suwon, Korea.
- 2Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Korea. paik@catholic.ac.kr
- KMID: 1434773
- DOI: http://doi.org/10.4167/jbv.2012.42.1.57
Abstract
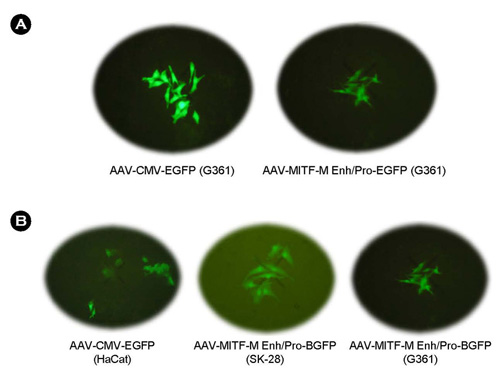
- We have developed the recombinant adeno-associated virus (AAV) carrying the EGFP gene under the control of the microphtalmia-associated transcription factor-M (MITF-M) promoter region for melanoma-specific expression. MITF-M distal enhancer (MDE) region enhances the specific expression of the reporter gene specifically in cultured melanoma cells. Expression of EGFP protein was very high in AAV-CMV-EGFP infected cells but relatively low in cells infected with AAV-Mitf(Enh/Pro)-EGFP. After an in vitro infection by a recombinant AAV carrying the EGFP gene under the control of human MITF-M promoter, the reporter gene was expressed in MITF-M producing melanoma cell lines (SK-28 and G361), but not in MITF-M non-producing cell lines (HaCat). These results suggest that the utilization of the MITF-M promoter in a recombinant AAV vector could provide benefits in gene therapy applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003. 46:546–555.
Article2. Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B, et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997. 8:1891–1900.
Article3. Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000. 97:13801–13806.
Article4. Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994. 8:148–154.
Article5. Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J, Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998. 72:10222–10226.
Article6. Yajima I, Sato S, Kimura T, Yasumoto K, Shibahara S, Goding CR, et al. An L1 element intronic insertion in the black-eyed white (Mitf[mi-bw]) gene: the loss of a single Mitf isoform responsible for the pigmentary defect and inner ear deafness. Hum Mol Genet. 1999. 8:1431–1441.
Article7. Watanabe K, Takeda K, Yasumoto K, Udono T, Saito H, Ikeda K, et al. Identification of a distal enhancer for the melanocyte-specific promoter of the MITF gene. Pigment Cell Res. 2002. 15:201–211.
Article8. Amae S, Fuse N, Yasumoto K, Sato S, Yajima I, Yamamoto H, et al. Identification of a novel isoform of microphthalmia-associated transcription factor that is enriched in retinal pigment epithelium. Biochem Biophys Res Commun. 1998. 247:710–715.
Article9. Fuse N, Yasumoto K, Suzuki H, Takahashi K, Shibahara S. Identification of a melanocyte-type promoter of the microphthalmia-associated transcription factor gene. Biochem Biophys Res Commun. 1996. 219:702–707.
Article10. Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988. 85:5976–5980.
Article11. Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986. 83:6682–6686.
Article12. Oliviero S, Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989. 8:1145–1151.
Article13. Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001. 75:8968–8976.
Article14. Gunzburg WH, Salmons B. Virus vector design in gene therapy. Mol Med Today. 1995. 1:410–417.
Article15. Dachs GU, Dougherty GJ, Stratford IJ, Chaplin DJ. Targeting gene therapy to cancer: A review. Oncol Res. 1997. 9:313–325.16. Baum C, Eckert HG, Stockschläder M, Just U, Hegewisch-Becker S, Hildinger M, et al. Improved retroviral vectors for hematopoietic stem cell protection and in vivo selection. J Hematother. 1996. 5:323–329.
Article17. Dunbar CE, Tisdale J, Yu JM, Soma T, Zujewski J, Bodine D, et al. Transduction of hematopoietic stem cells in humans and in nonhuman primates. Stem Cells. 1997. 15:135–139. discussion 139-40.
Article18. Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993. 4:403–409.
Article19. Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994. 91:6196–6200.
Article20. Park SW, Lee HK, Kim TG, Yoon SK, Paik SY. Hepatocyte-specific gene expression by baculovirus pseudotyped with vesicular stomatitis virus envelope glycoprotein. Biochem Biophys Res Commun. 2001. 289:444–450.
Article21. Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene Therapy Platform for Bone Regeneration Using an Exogenously Regulated, AAV-2-Based Gene Expression System. Mol Ther. 2004. 9:587–595.
Article22. Haberman RP, McCown TJ, Samulski RJ. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998. 5:1604–1611.
Article23. Fitzsimons HL, Mckenzie JM, During MJ. Insulators coupled to a minimal bidirectional tet cassette for tight regulation of rAAV-mediated gene transfer in the mammalian brain. Gene Ther. 2001. 8:1675–1681.
Article24. McGee Sanftner LH, Rendahl KG, Quiroz D, Coyne M, Ladner M, Manning WC, et al. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol Ther. 2001. 3:688–696.
Article25. Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003. 10:84–94.
Article26. Angeletti B, Löster J, Auricchio A, Gekeler F, Shinoda K, Ballabio A, et al. An in vivo doxycycline-controlled expression system for functional studies of the retina. Invest Ophthalmol Vis Sci. 2003. 44:755–760.
Article27. Haberman RP, McCown TJ. Regulation of gene expression in adeno-associated virus vectors in the brain. Methods. 2002. 28:219–226.
Article28. Johnston J, Tazelaar J, Rivera VM, Clackson T, Gao GP, Wilson JM. Regulated expression of erythropoietin from an AAV vector safely improves the anemia of beta-thalassemia in a mouse model. Mol Ther. 2003. 7:493–497.
Article29. Yang YW, Kotin RM. Glucose-responsive gene delivery in pancreatic islet cells via recombinant adeno-associated viral vectors. Pharm Res. 2000. 17:1056–1061.30. Jiang S, Altmann A, Grimm D, Kleinschmidt JA, Schilling T, Germann C, et al. Tissue-specific gene expression in medullary thyroid carcinoma cells employing calcitonin regulatory elements and AAV vectors. Cancer Gene Ther. 2001. 8:469–472.
Article31. Ruan H, Su H, Hu L, Lamborn KR, Kan YW, Deen DF. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001. 3:255–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of MITF-M and MITF-A Overexpression on the Dendrtic Formation in Melanocytes
- Constructtion of the Recombinant pAAVCMVp53 for Cervical Cancer Gene therapy
- Simple Purification of Adeno-Associated Virus-DJ for Liver-Specific Gene Expression
- Construction of a Mycobacterium - Escherichia coli Shuttle Vector and Use in the Expression of Foreign Genes in Mycobacteria
- Development of Tetracycline-regulated Adenovirus Expression Vector System