Int J Stem Cells.
2024 May;17(2):204-211. 10.15283/ijsc23071.
Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy
- Affiliations
-
- 1Stem Cell Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
- 2KRIBB School of Bioscience, Korea University of Science & Technology (UST), Daejeon, Korea
- KMID: 2556238
- DOI: http://doi.org/10.15283/ijsc23071
Abstract
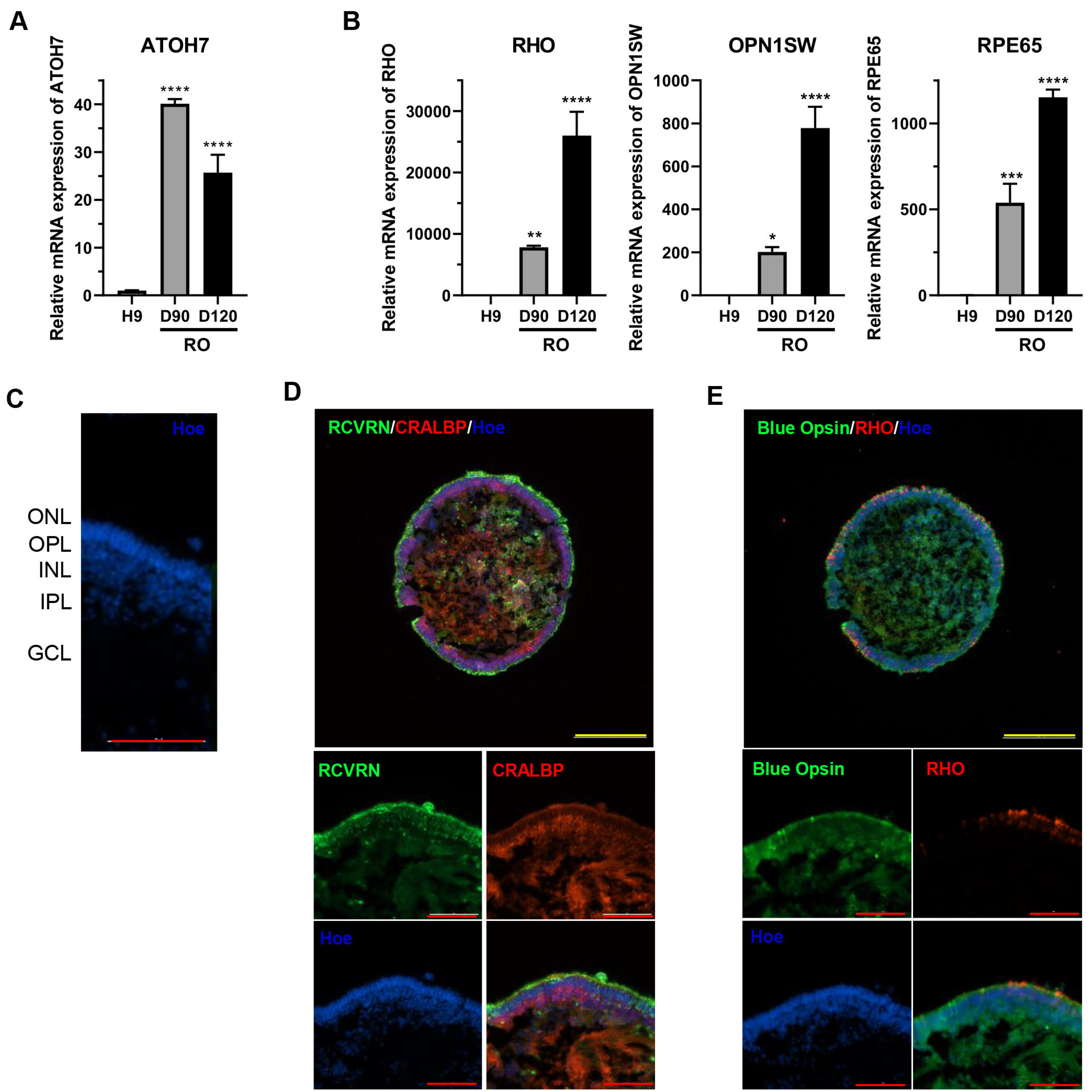
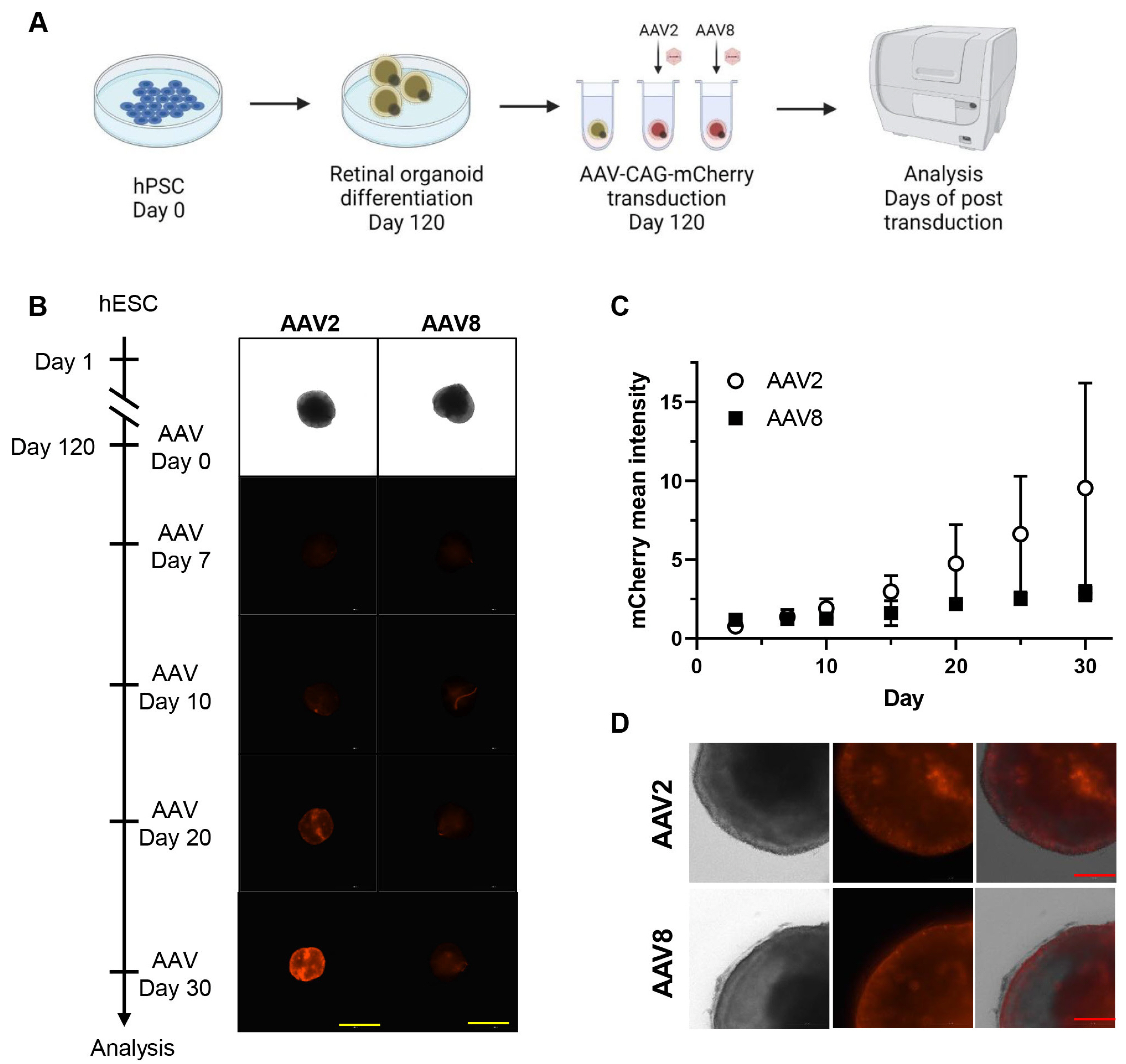
- With recent advances in adeno-associated virus (AAV)-based gene therapy, efficacy and toxicity screening have become essential for developing gene therapeutic drugs for retinal diseases. Retinal organoids from human pluripotent stem cells (hPSCs) offer a more accessible and reproducible human test platform for evaluating AAV-based gene therapy. In this study, hPSCs were differentiated into retinal organoids composed of various types of retinal cells. The transduction efficiencies of AAV2 and AAV8, which are widely used in clinical trials of inherited retinal diseases, were analyzed using retinal organoids. These results suggest that retinal organoids derived from hPSCs serve as suitable screening platforms owing to their diverse retinal cell types and similarity to the human retina. In summary, we propose an optimal stepwise protocol that includes the generation of retinal organoids and analysis of AAV transduction efficacy, providing a comprehensive approach for evaluating AAV-based gene therapy for retinal diseases.
Figure
Reference
-
References
1. Cideciyan AV, Hauswirth WW, Aleman TS, et al. 2009; Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 20:999–1004. DOI: 10.1089/hum.2009.086. PMID: 19583479. PMCID: PMC2829287.
Article2. Cukras C, Wiley HE, Jeffrey BG, et al. 2018; Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 26:2282–2294. DOI: 10.1016/j.ymthe.2018.05.025. PMID: 30196853. PMCID: PMC6127971.
Article3. Meng D, Ragi SD, Tsang SH. 2022; Therapy in rhodopsin-mediated autosomal dominant retinitis pigmentosa. Mol Ther. 30:2633. Erratum for: Mol Ther 2020;28:2139-2149. DOI: 10.1016/j.ymthe.2020.08.012. PMID: 32882181. PMCID: PMC7545001.
Article4. Wang D, Tai PWL, Gao G. 2019; Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 18:358–378. DOI: 10.1038/s41573-019-0012-9. PMID: 30710128. PMCID: PMC6927556.
Article5. Nakano T, Ando S, Takata N, et al. 2012; Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10:771–785. DOI: 10.1016/j.stem.2012.05.009. PMID: 22704518.
Article6. Zhong X, Gutierrez C, Xue T, et al. 2014; Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 5:4047. DOI: 10.1038/ncomms5047. PMID: 24915161. PMCID: PMC4370190.
Article7. Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, Sasai Y. 2015; Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun. 6:6286. DOI: 10.1038/ncomms7286. PMID: 25695148.
Article8. Cowan CS, Renner M, De Gennaro M, et al. 2020; Cell types of the human retina and its organoids at single-cell resolution. Cell. 182:1623–1640.e34. DOI: 10.1016/j.cell.2020.08.013. PMID: 32946783. PMCID: PMC7505495.
Article9. Finkbeiner C, Ortuño-Lizarán I, Sridhar A, Hooper M, Petter S, Reh TA. 2022; Single-cell ATAC-seq of fetal human retina and stem-cell-derived retinal organoids shows changing chromatin landscapes during cell fate acquisition. Cell Rep. 38:110294. DOI: 10.1016/j.celrep.2021.110294. PMID: 35081356.
Article10. Saha A, Capowski E, Fernandez Zepeda MA, Nelson EC, Gamm DM, Sinha R. 2022; Cone photoreceptors in human stem cell-derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell. 29:460–471.e3. Erratum in: Cell Stem Cell 2022;29:487-489. DOI: 10.1016/j.stem.2022.02.003. PMID: 35245468. PMCID: PMC9119348.
Article11. Kwon OS, Na HJ, Ahn J, Chung KS. 2022; Establishment of a human induced pluripotent stem cell line, KRIBBi006-A, from peripheral blood mononuclear cells derived from a healthy male donor. Stem Cell Res. 65:102950. DOI: 10.1016/j.scr.2022.102950. PMID: 36283271.
Article12. Zou C, Levine EM. 2012; Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet. 8:e1002924. DOI: 10.1371/journal.pgen.1002924. PMID: 23028343. PMCID: PMC3447932.
Article13. Xie H, Zhang W, Zhang M, et al. 2020; Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Sci Adv. 6:eaay5247. DOI: 10.1126/sciadv.aay5247. PMID: 32083182. PMCID: PMC7007246.
Article14. Garita-Hernandez M, Routet F, Guibbal L, et al. 2020; AAV-mediated gene delivery to 3D retinal organoids derived from human induced pluripotent stem cells. Int J Mol Sci. 21:994. DOI: 10.3390/ijms21030994. PMID: 32028585. PMCID: PMC7036814.
Article15. Lane A, Jovanovic K, Shortall C, et al. 2020; Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Reports. 15:67–79. DOI: 10.1016/j.stemcr.2020.05.007. PMID: 32531192. PMCID: PMC7363745.
Article16. Völkner M, Pavlou M, Büning H, Michalakis S, Karl MO. 2021; Optimized adeno-associated virus vectors for efficient transduction of human retinal organoids. Hum Gene Ther. 32:694–706. DOI: 10.1089/hum.2020.321.
Article17. Vandenberghe LH, Bell P, Maguire AM, et al. 2011; Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 3:88ra54. Erratum in: Sci Transl Med 2011;3:112er9. DOI: 10.1126/scitranslmed.3002103. PMID: 21697530. PMCID: PMC5027886.
Article18. Surace EM, Auricchio A. 2008; Versatility of AAV vectors for retinal gene transfer. Vision Res. 48:353–359. DOI: 10.1016/j.visres.2007.07.027. PMID: 17923143.
Article19. Achberger K, Cipriano M, Düchs MJ, et al. 2021; Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors. Stem Cell Reports. 16:2242–2256. DOI: 10.1016/j.stemcr.2021.08.008. PMID: 34525384. PMCID: PMC8452599.
Article20. Byrne LC, Day TP, Visel M, et al. 2020; In vivo-directed evolution of adeno-associated virus in the primate retina. JCI Insight. 5:e135112. DOI: 10.1172/jci.insight.135112. PMID: 32271719. PMCID: PMC7259523.
Article21. Cazier AP, Blazeck J. 2021; Advances in promoter engineering: novel applications and predefined transcriptional control. Biotechnol J. 16:e2100239. DOI: 10.1002/biot.202100239. PMID: 34351706.
Article22. Pan X, Yue Y, Boftsi M, et al. 2022; Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 29:333–345. DOI: 10.1038/s41434-021-00296-0. PMID: 34611321. PMCID: PMC8983793.
Article23. Fligor CM, Lavekar SS, Harkin J, et al. 2021; Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Reports. 16:2228–2241. DOI: 10.1016/j.stemcr.2021.05.009. PMID: 34115986. PMCID: PMC8452489.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Vascularized Organoids: A More Complete Model
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Applications of kidney organoids derived from human pluripotent stem cells