Int J Stem Cells.
2022 Feb;15(1):95-103. 10.15283/ijsc21195.
A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Affiliations
-
- 1Department of Stem Cell and Regenerative Biotechnology, KU Institute of Science and Technology, Konkuk University, Seoul, Korea
- KMID: 2526741
- DOI: http://doi.org/10.15283/ijsc21195
Abstract
- Background and Objectives
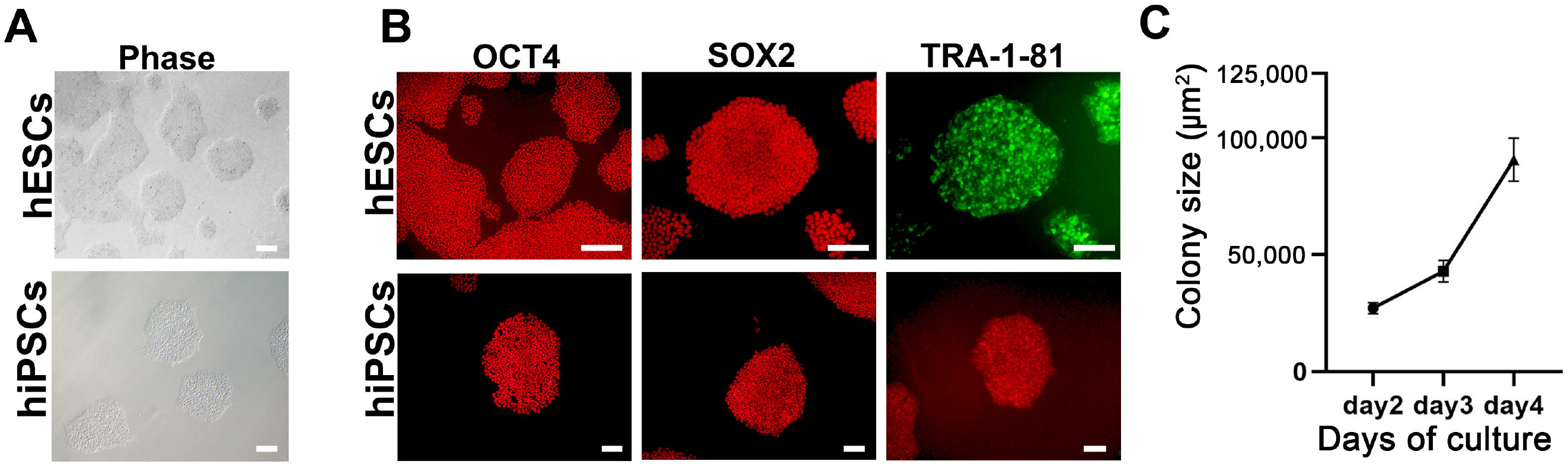
In recent years, brain organoid technologies have been the most innovative advance in neural differentiation research. In line with this, we optimized a method to establish cerebral organoids from feeder-free cultured human pluripotent stem cells. In this study, we focused on the consistent and robust production of cerebral organoids comprising neural progenitor cells and neurons. We propose an optimal protocol for cerebral organoid generation that is applicable to both human embryonic stem cells and human induced pluripotent stem cells.
Methods and Results
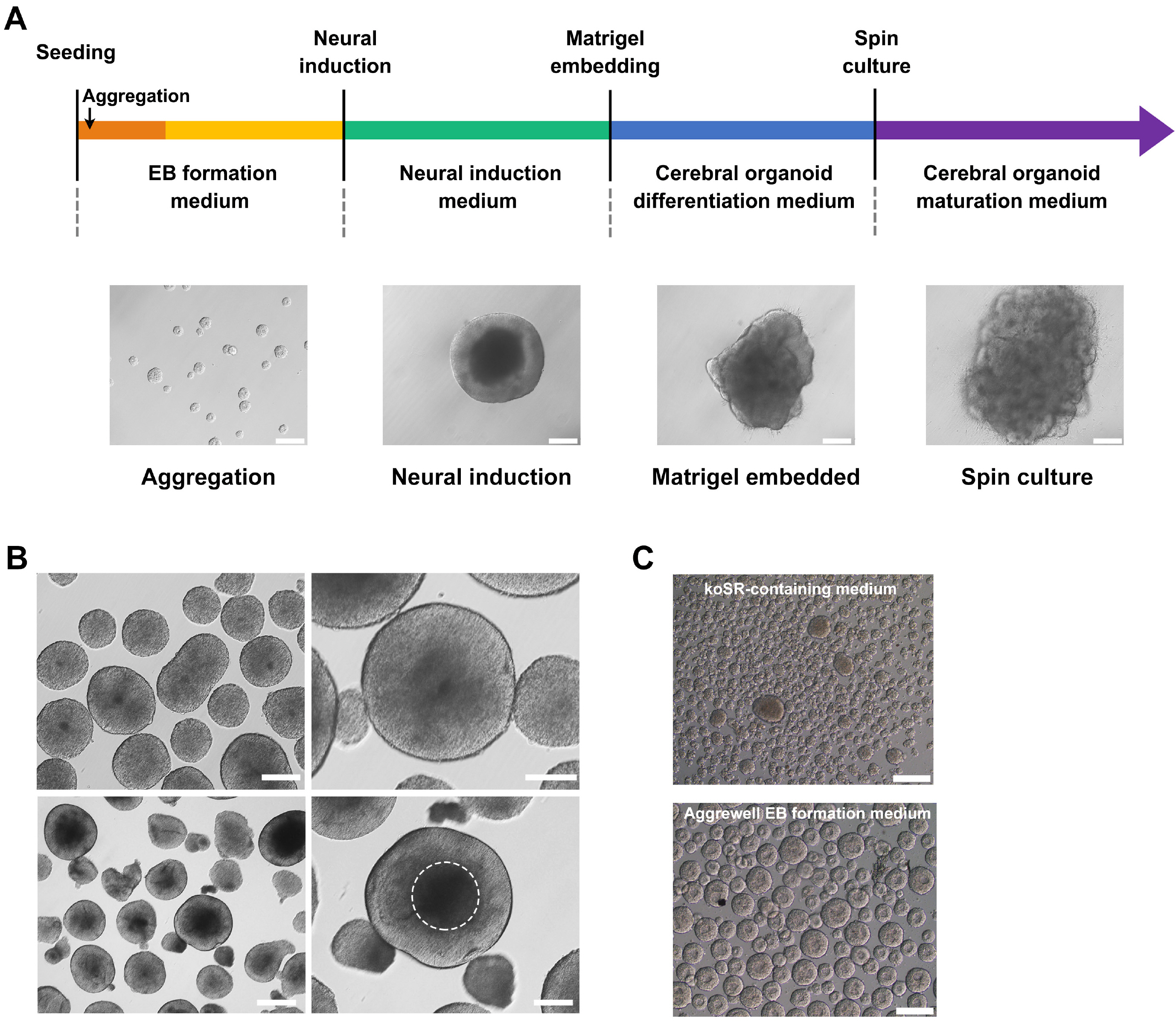
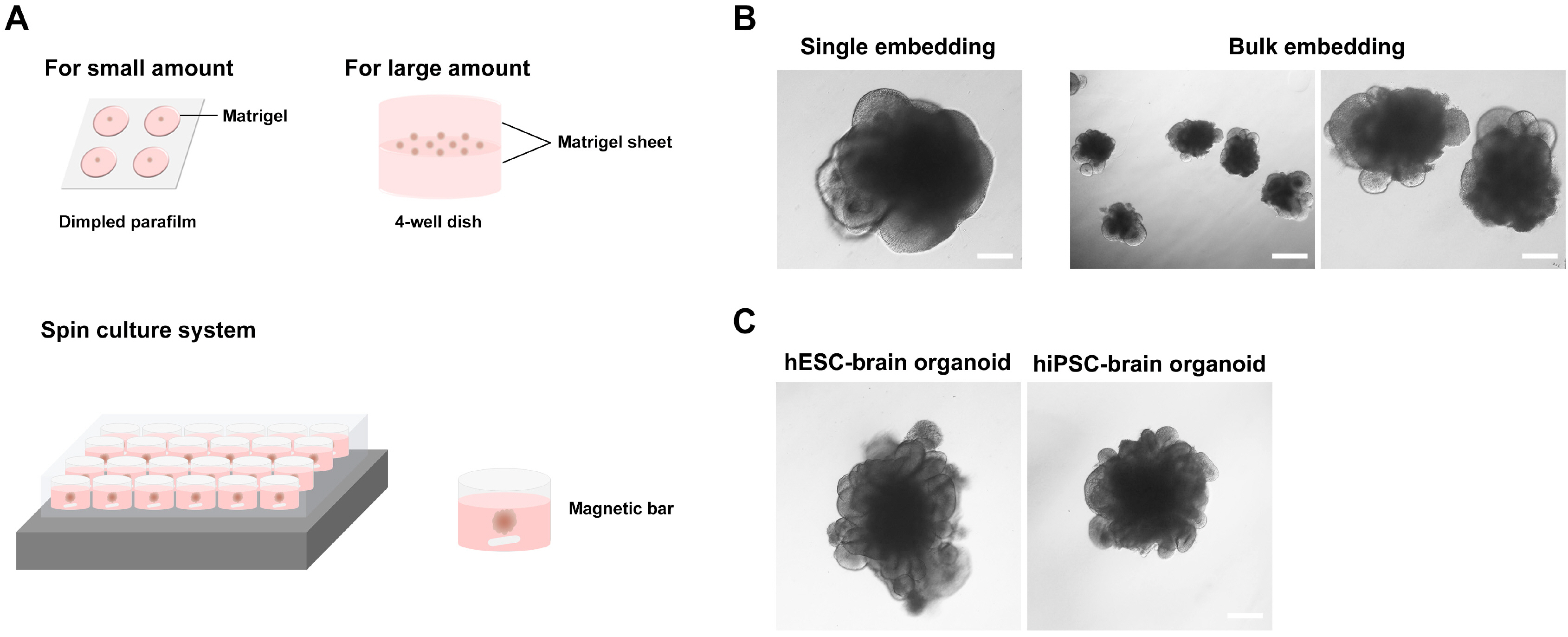
We investigated formation of neuroepithelium, neural tube, and neural folding by observing the morphology of embryoid bodies at each stage during the cerebral organoid differentiation process. Furthermore, we characterized the cerebral organoids via immunocytochemical staining of sectioned organoid samples, which were prepared using a Cryostat and Vibratome. Finally, we established a routine method to generate early cerebral organoids comprising a cortical layer and a neural progenitor zone.
Conclusions
We developed an optimized methodology for the generation of cerebral organoids using hESCs and hiPSCs. Using this protocol, consistent and efficient cerebral organoids could be obtained from hiPSCs as well as hESCs. Further, the morphology of brain organoids could be analyzed through 2D monitoring via immunostaining and tissue sectioning, or through 3D monitoring by whole tissue staining after clarification.
Figure
Cited by 2 articles
-
Lo and Behold, the Lab-Grown Organs Have Arrived!
Jaesang Kim
Int J Stem Cells. 2022;15(1):1-2. doi: 10.15283/ijsc22026.Guidelines for Manufacturing and Application of Organoids: Brain
Taehwan Kwak, Si-Hyung Park, Siyoung Lee, Yujeong Shin, Ki-Jun Yoon, Seung-Woo Cho, Jong-Chan Park, Seung-Ho Yang, Heeyeong Cho, Heh-In Im, Sun-Ju Ahn, Woong Sun, Ji Hun Yang
Int J Stem Cells. 2024;17(2):158-181. doi: 10.15283/ijsc24056.
Reference
-
References
1. Fatehullah A, Tan SH, Barker N. 2016; Organoids as an in vitro model of human development and disease. Nat Cell Biol. 18:246–254. DOI: 10.1038/ncb3312. PMID: 26911908.
Article2. Kelava I, Lancaster MA. 2016; Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev Biol. 420:199–209. DOI: 10.1016/j.ydbio.2016.06.037. PMID: 27402594. PMCID: PMC5161139.
Article3. Di Lullo E, Kriegstein AR. 2017; The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 18:573–584. DOI: 10.1038/nrn.2017.107. PMID: 28878372. PMCID: PMC5667942.
Article4. Quadrato G, Arlotta P. 2017; Present and future of modeling human brain development in 3D organoids. Curr Opin Cell Biol. 49:47–52. DOI: 10.1016/j.ceb.2017.11.010. PMID: 29227864.
Article5. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI: 10.1038/nature12517. PMID: 23995685. PMCID: PMC3817409.
Article6. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. 2017; Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 545:48–53. DOI: 10.1038/nature22047. PMID: 28445462. PMCID: PMC5659341.
Article7. Qian X, Song H, Ming GL. 2019; Brain organoids: advances, applications and challenges. Development. 146:dev166074. DOI: 10.1242/dev.166074. PMID: 30992274. PMCID: PMC6503989.
Article8. Nam KH, Yi SA, Jang HJ, Han JW, Lee J. 2020; In vitro modeling for inherited neurological diseases using induced pluripotent stem cells: from 2D to organoid. Arch Pharm Res. 43:877–889. DOI: 10.1007/s12272-020-01260-z. PMID: 32761309.
Article9. Koo B, Choi B, Park H, Yoon KJ. 2019; Past, present, and future of brain organoid technology. Mol Cells. 42:617–627. DOI: 10.14348/molcells.2019.0162. PMID: 31564073. PMCID: PMC6776157.10. Hong YJ, Do JT. 2019; Neural lineage differentiation from pluripotent stem cells to mimic human brain tissues. Front Bioeng Biotechnol. 7:400. DOI: 10.3389/fbioe.2019.00400. PMID: 31867324. PMCID: PMC6908493. PMID: b6a24945b52a4791bcc928625ce33ea3.
Article11. Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. 2001; In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 19:1129–1133. DOI: 10.1038/nbt1201-1129. PMID: 11731781.
Article12. Lancaster MA, Knoblich JA. 2014; Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 9:2329–2340. DOI: 10.1038/nprot.2014.158. PMID: 25188634. PMCID: PMC4160653.
Article13. Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. 2013; Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 110:20284–20289. Erratum in: Proc Natl Acad Sci U S A 2014;111:7498. DOI: 10.1073/pnas.1315710110. PMID: 24277810. PMCID: PMC3864329.
Article14. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. 2016; Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 165:1238–1254. DOI: 10.1016/j.cell.2016.04.032. PMID: 27118425. PMCID: PMC4900885.
Article15. Giandomenico SL, Sutcliffe M, Lancaster MA. 2021; Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat Protoc. 16:579–602. DOI: 10.1038/s41596-020-00433-w. PMID: 33328611. PMCID: PMC7611064.
Article16. Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, Nguyen PTT, Huh S, Hoke A, Swinford-Jackson SE, Wen Z, Gu X, Pierce RC, Wu H, Briand LA, Chen HI, Wolf JA, Song H, Ming GL. 2020; Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 26:766–781.e9. DOI: 10.1016/j.stem.2020.02.002. PMID: 32142682. PMCID: PMC7366517.
Article17. Chung K, Deisseroth K. 2013; CLARITY for mapping the nervous system. Nat Methods. 10:508–513. Erratum in: Nat Methods 2013;10:1035. DOI: 10.1038/nmeth.2481. PMID: 23722210.
Article18. Azaripour A, Lagerweij T, Scharfbillig C, Jadczak AE, Willershausen B, Van Noorden CJ. 2016; A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog Histochem Cytochem. 51:9–23. DOI: 10.1016/j.proghi.2016.04.001. PMID: 27142295.
Article19. Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP. 2017; Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 95:779–790.e6. DOI: 10.1016/j.neuron.2017.07.035. PMID: 28817799. PMCID: PMC5890820.
Article20. Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pașca SP. 2019; Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 22:484–491. DOI: 10.1038/s41593-018-0316-9. PMID: 30692691. PMCID: PMC6788758.
Article21. Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, Kaplitt M, Neff C, Rapalino O, Seo H, Lee IH, Kim J, Kim T, Petsko GA, Ritz J, Cohen BM, Kong SW, Leblanc P, Carter BS, Kim KS. 2020; Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 382:1926–1932. DOI: 10.1056/NEJMoa1915872. PMID: 32402162. PMCID: PMC7288982.
Article22. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. 2009; Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 27:275–280. Erratum in: Nat Biotechnol 2009;27:485. DOI: 10.1038/nbt.1529. PMID: 19252484. PMCID: PMC2756723.
Article23. Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, Jarazo J, Walter J, Brüggemann I, Boussaad I, Berger E, Fleming RMT, Bolognin S, Schwamborn JC. 2017; Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Reports. 8:1144–1154. DOI: 10.1016/j.stemcr.2017.03.010. PMID: 28416282. PMCID: PMC5425618.
Article24. Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, Kong SW, Schweitzer JS, Kim KS. 2020; Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models. J Clin Invest. 130:904–920. DOI: 10.1172/JCI130767. PMID: 31714896. PMCID: PMC6994130.
Article25. Shaker MR, Pietrogrande G, Martin S, Lee JH, Sun W, Wolvetang EJ. 2021; Rapid and efficient generation of myelinating human oligodendrocytes in organoids. Front Cell Neurosci. 15:631548. DOI: 10.3389/fncel.2021.631548. PMID: 33815061. PMCID: PMC8010307. PMID: f2310921ad594e789e3e1009cf8d45c1.
Article26. Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. 2018; Microglia innately develop within cerebral organoids. Nat Commun. 9:4167. DOI: 10.1038/s41467-018-06684-2. PMID: 30301888. PMCID: PMC6177485. PMID: 0f43c1e80fff44ada197169a4b75a7dc.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cerebral organoid research for pediatric patients with neurological disorders
- Vascularized Organoids: A More Complete Model
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy