Hanyang Med Rev.
2015 Nov;35(4):196-206. 10.7599/hmr.2015.35.4.196.
Recent Advances for Enhancing Drug Metabolizing Functions of Hepatocyte-like Cells Derived from Human Pluripotent Stem Cells
- Affiliations
-
- 1Laboratory of Stem Cells and Tissue Regeneration, Department of Biotechnology, College of Life Sciences and Biotechnology, Science Campus, Korea University, Seoul, Korea. jhkim@korea.ac.kr
- KMID: 2129472
- DOI: http://doi.org/10.7599/hmr.2015.35.4.196
Abstract
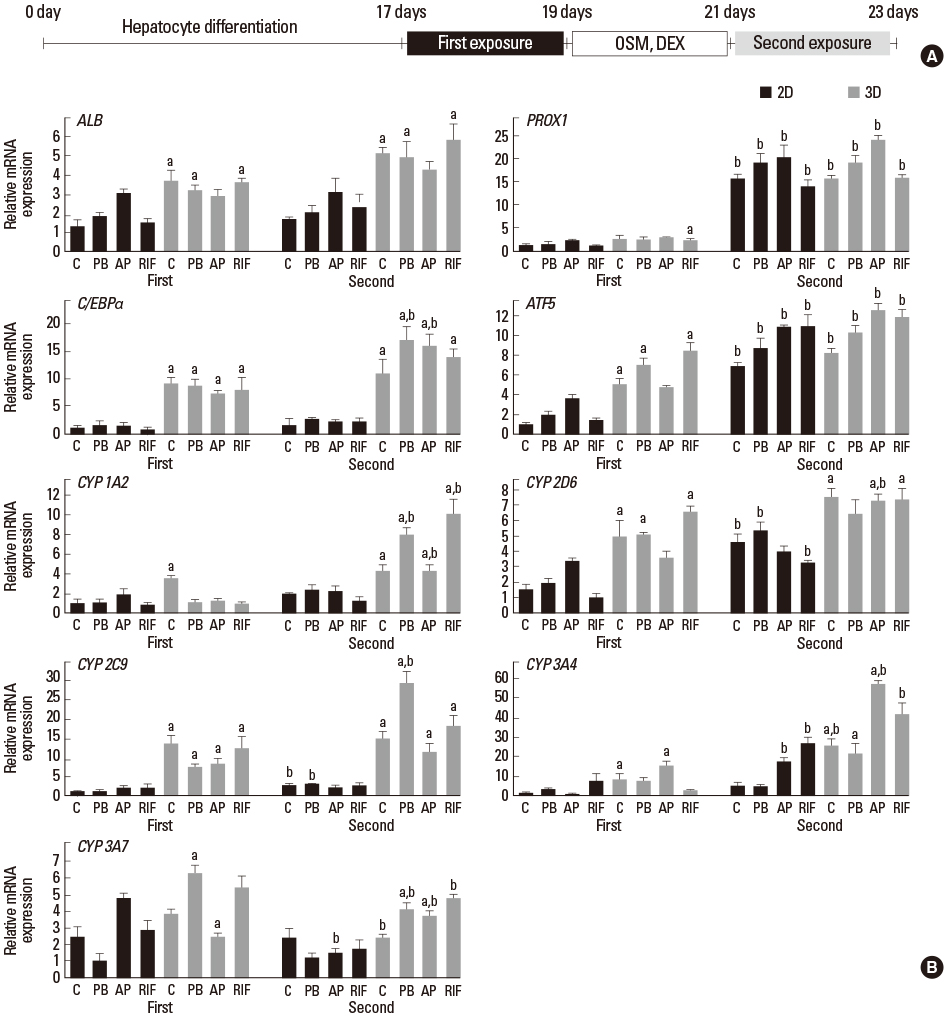
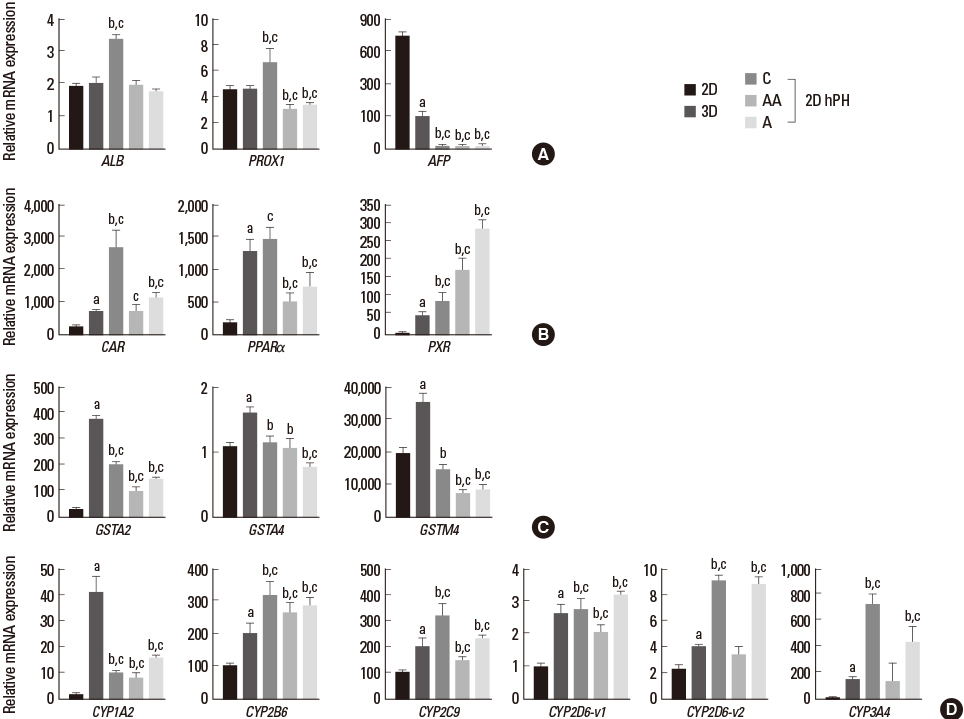
- Hepatocyte-like cells (HLCs) derived from human pluripotent stem cells are a promising cell source for drug screening and toxicity tests. Thus, various hepatic differentiating protocols have been developed, leading to a hepatic differentiation efficiency of approximately 90%. However, HLC drug metabolizing ability remains very low compared to human primary hepatocytes. In order to overcome this problem, several alternative methods, such as, co-culture, three-dimensional (3D) culture, bioreactor, nanochip-based, etc., have been developed, but optimization to produce fully functional HLCs is ongoing. Recently, our group reported that repeated exposure of HLCs to xenobiotics can improve the expression of hepatic metabolizing enzymes such as cytochrome P450s (CYPs) and glutathione S-transferases (GSTs). These data suggest that we should develop strategies for differentiating cells into mature HLCs by more closely mimicking in vivo fetal and postnatal liver development. Here, we review the current development of alternative methods for enhancing the drug metabolizing functions of HLCs derived from human embryonic stem cells, human-induced pluripotent stem cells, and mesenchymal stem cells as used for drug screening and toxicity tests.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
New Horizons in Stem Cell Research
Dongho Choi
Hanyang Med Rev. 2015;35(4):187-189. doi: 10.7599/hmr.2015.35.4.187.
Reference
-
1. Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, et al. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol. 2013; 75:885–896.
Article2. Kim JH, Jang YJ, An SY, Son J, Lee J, Lee G, et al. Enhanced metabolizing activity of human ES cell-derived hepatocytes using a 3D culture system with repeated exposures to xenobiotics. Toxicol Sci. 2015; 147:190–206.
Article3. Subramanian K, Owens DJ, Raju R, Firpo M, O'Brien TD, Verfaillie CM, et al. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014; 23:124–131.
Article4. The world's first commercial human iPSC-derived hepatocytes [Internet]. Yokohama (JPN): ReproCELL;c2012. cited 2015 Oct 20. Available from: https://www.reprocell.com/en/products/cell-biorepository/reprohepato/.5. Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014; 32:504–513.
Article6. Meng Q, Haque A, Hexig B, Akaike T. The differentiation and isolation of mouse embryonic stem cells toward hepatocytes using galactose-carrying substrata. Biomaterials. 2012; 33:1414–1427.
Article7. Fair JH, Cairns BA, Lapaglia M, Wang J, Meyer AA, Kim H, et al. Induction of hepatic differentiation in embryonic stem cells by co-culture with embryonic cardiac mesoderm. Surgery. 2003; 134:189–196.
Article8. LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012; 42:501–548.
Article9. Neuman MG, Brenner DA, Rehermann B, Taieb J, Chollet-Martin S, Cohard M, et al. Mechanisms of alcoholic liver disease: cytokines. Alcohol Clin Exp Res. 2001; 25:251S–253S.
Article10. Ishii T, Yasuchika K, Fukumitsu K, Kawamoto T, Kawamura-Saitoh M, Amagai Y, et al. In vitro hepatic maturation of human embryonic stem cells by using a mesenchymal cell line derived from murine fetal livers. Cell Tissue Res. 2010; 339:505–512.
Article11. Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology. 2015; 61:1370–1381.
Article12. Setty Y, Cohen IR, Dor Y, Harel D. Four-dimensional realistic modeling of pancreatic organogenesis. Proc Natl Acad Sci U S A. 2008; 105:20374–20379.
Article13. Reid LM, Gaitmaitan Z, Arias I, Ponce P, Rojkind M. Long-term cultures of normal rat hepoatocytes on liver biomatrix. Ann N Y Acad Sci. 1980; 349:70–76.14. Park HJ, Choi YJ, Kim JW, Chun HS, Im I, Yoon S, et al. Differences in the epigenetic regulation of cytochrome P450 genes between human embryonic stem cell-derived hepatocytes and primary hepatocytes. PLoS One. 2015; 10:e0132992.
Article15. Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports. 2015; 4:939–952.
Article16. Buyl K, De Kock J, Najar M, Lagneaux L, Branson S, Rogiers V, et al. Characterization of hepatic markers in human Wharton's Jelly-derived mesenchymal stem cells. Toxicol In Vitro. 2014; 28:113–119.
Article17. Farzaneh Z, Pakzad M, Vosough M, Pournasr B, Baharvand H. Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem Cell Biol. 2014; 142:217–226.
Article18. Vaghjiani V, Vaithilingam V, Saraswati I, Sali A, Murthi P, Kalionis B, et al. Hepatocyte-like cells derived from human amniotic epithelial cells can be encapsulated without loss of viability or function in vitro. Stem Cells Dev. 2014; 23:866–876.
Article19. Subramanian K, Owens DJ, Raju R, Firpo M, O'Brien TD, Verfaillie CM, et al. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014; 23:124–131.
Article20. Park Y, Chen Y, Ordovas L, Verfaillie CM. Hepatic differentiation of human embryonic stem cells on microcarriers. J Biotechnol. 2014; 174:39–48.
Article21. Li X, Yuan J, Li W, Liu S, Hua M, Lu X, et al. Direct differentiation of homogeneous human adipose stem cells into functional hepatocytes by mimicking liver embryogenesis. J Cell Physiol. 2014; 229:801–812.
Article22. Gieseck RL, Hannan NR, Bort R, Hanley NA, Drake RA, Cameron GW, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014; 9:e86372.
Article23. Kondo Y, Iwao T, Nakamura K, Sasaki T, Takahashi S, Kamada N, et al. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab Pharmacokinet. 2014; 29:237–243.
Article24. Ulvestad M, Nordell P, Asplund A, Rehnström M, Jacobsson S, Holmgren G, et al. Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol. 2013; 86:691–702.
Article25. Yanagida A, Ito K, Chikada H, Nakauchi H, Kamiya A. An in vitro expansion system for generation of human iPS cell-derived hepatic progenitor-like cells exhibiting a bipotent differentiation potential. PLoS One. 2013; 8:e67541.
Article26. Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med. 2013; 2:409–419.
Article27. Cheong HH, Masilamani J, Chan CY, Chan SY, Phan TT. Metabolically functional hepatocyte-like cells from human umbilical cord lining epithelial cells. Assay Drug Dev Technol. 2013; 11:130–138.
Article28. Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013; 14:961–972.
Article29. Dong X, Pan R, Zhang H, Yang C, Shao J, Xiang L. Modification of histone acetylation facilitates hepatic differentiation of human bone marrow mesenchymal stem cells. PLoS One. 2013; 8:e63405.
Article30. Sgodda M, Mobus S, Hoepfner J, Sharma AD, Schambach A, Greber B, et al. Improved hepatic differentiation strategies for human induced pluripotent stem cells. Curr Mol Med. 2013; 13:842–855.
Article31. Yang G, Si-Tayeb K, Corbineau S, Vernet R, Gayon R, Dianat N, et al. Integration-deficient lentivectors: an effective strategy to purify and differentiate human embryonic stem cell-derived hepatic progenitors. BMC Biol. 2013; 11:86.
Article32. Sasaki T, Takahashi S, Numata Y, Narita M, Tanaka Y, Kumagai T, et al. Hepatocyte nuclear factor 6 activates the transcription of CYP3A4 in hepatocyte-like cells differentiated from human induced pluripotent stem cells. Drug Metab Pharmacokinet. 2013; 28:250–259.
Article33. Seeliger C, Culmes M, Schyschka L, Yan X, Damm G, Wang Z, et al. Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: possible future application for urea detoxification. Cell Transplant. 2013; 22:119–131.
Article34. Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013; 268:1–16.
Article35. Ramasamy TS, Yu JS, Selden C, Hodgson H, Cui W. Application of three-dimensional culture conditions to human embryonic stem cell-derived definitive endoderm cells enhances hepatocyte differentiation and functionality. Tissue Eng Part A. 2013; 19:360–367.
Article36. Vosough M, Omidinia E, Kadivar M, Shokrgozar MA, Pournasr B, Aghdami N, et al. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013; 22:2693–2705.
Article37. Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012; 55:1193–1203.
Article38. Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, et al. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Res. 2012; 9:196–207.
Article39. Hua M, Zhang W, Li W, Li X, Liu B, Lu X, et al. Molecular mechanisms regulating the establishment of hepatocyte polarity during human hepatic progenitor cell differentiation into a functional hepatocyte-like phenotype. J Cell Sci. 2012; 125:5800–5810.
Article40. Kim SE, An SY, Woo DH, Han J, Kim JH, Jang YJ, et al. Engraftment potential of spheroid-forming hepatic endoderm derived from human embryonic stem cells. Stem cells Dev. 2013; 22:1818–1829.
Article41. Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012; 142:602–611.
Article42. Wang D, Liu W, Han B, Xu R. The bioreactor: a powerful tool for large-scale culture of animal cells. Curr Pharm Biotechnol. 2005; 6:397–403.
Article43. Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K. Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A. 2010; 4:3285–3298.
Article44. Yoffe B, Darlington GJ, Soriano HE, Krishnan B, Risin D, Pellis NR, et al. Cultures of human liver cells in simulated microgravity environment. Adv Space Res. 1999; 24:829–836.
Article45. iCell® Hepatocytes [Internet]. USA: Cellular dynamics international;c2013. cited 2015 Oct 20. Available from: http://cellulardynamics.com/products-services/icell-products/icell-hepatocytes/.46. Stem cell techonology: liversafe 3DTM [Internet]. San Francisco (CA): VistaGen Therapeutics;cited 2015 Oct 20. Available from: http://www.vistagen.com/?page_id=113/.47. Stem assays: hepatoGLOTM [Internet]. Colorado Springs (CO): Hemo Genix Changing the Pradigm;cited 2015 Oct 20. Available from: http://www.hemogenix.com/prod_STEM1.php?tab=6/.48. Geron. [Internet]. cited 2015 Oct 20. Available from: http://www.geron.com/.49. Stem cell research: human stem cell derived hepatocytes [Internet]. Otsu (JPN): TaKaRa, Clontech;cited 2015 Oct 20. Available from: http://www.cellartis.com/stem-cell-research/.50. DiMasi JA. The value of improving the productivity of the drug development process: faster times and better decisions. Pharmacoeconomics. 2002; 20:Suppl 3. 1–10.
Article51. Chapman KL, Holzgrefe H, Black LE, Brown M, Chellman G, Copeman C, et al. Pharmaceutical toxicology: designing studies to reduce animal use, while maximizing human translation. Regul Toxicol Pharmacol. 2013; 66:88–103.
Article52. Hutchinson L, Kirk R. High drug attrition rates--where are we going wrong? Nat Rev Clin Oncol. 2011; 8:189–190.
Article53. Potta SP, Šarić T, Heke M, Bahudhanapati H, Hescheler J. Human Pluripotent stem cell applications in drug discovery and toxicology - an overview. In : Atwood CS, Meethal SV, editors. Pluripotent stem cell biology - advances in mechanisms, methods and models. Atlanta: Intech;2014. p. 181–196.54. Wanless IR. Anatomy, histology, embryology, and developmental anomalies of the liver. In : Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran's Gastrointestinal and Liver Disease. 7th ed. Philadelphia: Saunders;2002. p. 1195–1201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cells in Drug Screening for Neurodegenerative Disease
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- New Advances in Human X Chromosome Status from a Developmental and Stem Cell Biology
- Modeling of Human Genetic Diseases Via Cellular Reprogramming
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro