J Korean Med Sci.
2005 Feb;20(1):82-87. 10.3346/jkms.2005.20.1.82.
Gene Expression Analysis of Cultured Amniotic Fluid Cell with Down Syndrome by DNA Microarray
- Affiliations
-
- 1Functional Genomics Lab, Bundang Campus, College of Medicine, Pochon CHA University, Sungnam, Korea. suman@cha.ac.kr
- 2Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3The Center for Functional Analysis of Human Genome, KRIBB, Daejeon, Korea.
- KMID: 1781731
- DOI: http://doi.org/10.3346/jkms.2005.20.1.82
Abstract
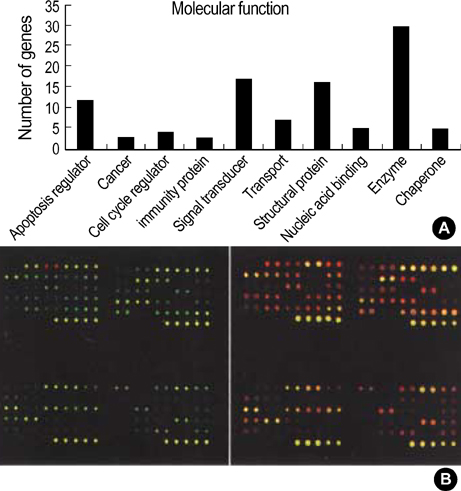
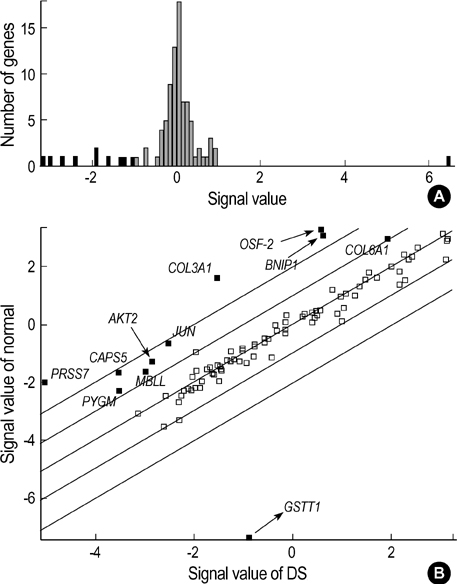
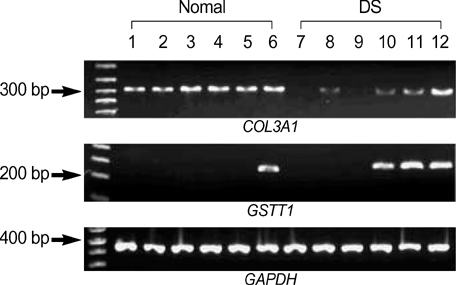
- Complete or partial triplication of human chromosome 21 results in Down syndrome (DS). To analyze differential gene expressions in amniotic fluid (AF) cells of DS, we used a DNA microarray system to analyze 102 genes, which included 24 genes on chromosome 21, 28 genes related to the function of brain and muscle, 36 genes related to apoptosis, 4 genes related to extracellular matrix, 8 genes related to other molecular function and 2 house-keeping genes. AF cells were collected from 12 pregnancies at 16-18 weeks of gestation in DS (n=6) and normal (n=6) subjects. Our DNA microarray experiments showed that the expressions of 11 genes were altered by at least 2-folds in DS, as follows. Ten genes, COL6A1, CASP5, AKT2, JUN, PYGM, BNIP1, OSF-2, PRSS7, COL3A1, and MBLL were down-regulated and GSTT1 was only up-regulated. The differential expressions of GSTT1 and COL3A1 were further confirmed by semi-quantitative RT-PCR for each sample. The gene dosage hypothesis on chromosome 21 may explain the neurological and other symptoms of DS. However, our results showed that only two genes (COL6A1 and PRSS7), among 24 genes on chromosome 21, were down-regulated in the AF cells of DS. Our data may provide the basis for a more systematic identification of biological markers of fetal DS, thus leading to an improved understanding of pathogenesis for fetal DS.
MeSH Terms
-
Amniocentesis
Amniotic Fluid/*cytology/*metabolism
Apoptosis
Cells, Cultured
Chromosomes, Human, Pair 21
Collagen Type III/biosynthesis
DNA, Complementary/metabolism
Down Syndrome/*genetics/metabolism
Down-Regulation
Gene Dosage
Gene Expression
*Gene Expression Regulation
Glutathione Transferase/biosynthesis
Humans
Models, Genetic
*Oligonucleotide Array Sequence Analysis
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Time Factors
Up-Regulation
Figure
Reference
-
1. Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, Carpenter N, Daumer C, Dignan P, Disteche C, Graham JM Jr, Hudgins L, Mcgillivray B, Miyazaki K, Ogasawara N, Park JP, Pagon R, Pueschel S, Sack G, Say B, Schuffenhauer S, Soukup S, Yamanaka T. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA. 1994. 91:4997–5001.
Article2. Lamb NE, Feingold E, Savage A, Avramopoulos D, Freeman S, Gu Y, Hallberg A, Hersey J, Karadima G, Pettay D, Saker D, Shen J, Taft L, Mikkelsen M, Petersen MB, Hassold T, Sherman SL. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum Mol Genet. 1997. 6:1391–1399.
Article3. Antonarakis SE. 10 years of Genomics, chromosome 21, and Down syndrome. Genomics. 1998. 51:1–16.4. Woodhouse JM, Hodge SJ, Earlam RA. Facial characteristics in children with Down's syndrome and spectacle fitting. Ophthalmic Physiol Opt. 1994. 14:25–31.
Article5. Engidawork E, Gulesserian T, Fountoulakis M, Lubec G. Aberrant protein expression in cerebral cortex of fetus with Down syndrome. Neuroscience. 2003. 122:145–154.
Article6. Takashima S, Becker LE, Armstrong DL, Chan F. Abnormal neuronal development in the visual cortex of the human fetus and infant with Down's syndrome. A quantitative and qualitative Golgi study. Brain Res. 1981. 225:1–21.
Article7. Ferencz C, Neill CA, Boughman JA, Rubin JD, Brenner JI, Perry LW. Congenital cardiovascular malformations associated with chromosome abnormalities: an epidemiologic study. J Pediatr. 1989. 114:79–86.
Article8. Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, Khoury MJ, Saker DM. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998. 80:213–217.
Article9. Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000. 9:1681–1690.
Article10. Yamaki A, Tochigi J, Kudoh J, Minoshima S, Shimizu N, Shimizu Y. Molecular mechanisms of human single-minded 2 (SIM2) gene expression: identification of a promoter site in the SIM2 genomic sequence. Gene. 2001. 270:265–275.
Article11. Griffin WS, Sheng JG, McKenzie JE, Royston MC, Gentleman SM, Brumback RA, Cork LC, Del Bigio MR, Roberts GW, Mrak RE. Life-long overexpression of S100beta in Down's syndrome: implications for Alzheimer pathogenesis. Neurobiol Aging. 1998. 19:401–405.12. Roh MS, Hong SH, Jeong JS, Kwon HC, Kim MC, Cho SH, Yoon JH, Hwang TH. Gene expression profiling of breast cancers with emphasis of beta-catenin regulation. J Korean Med Sci. 2004. 19:275–282.13. Prusa AR, Marton E, Rosner M, Freilinger A, Bernaschek G, Hengstschlager M. Stem cell marker expression in human trisomy 21 amniotic fluid cells and trophoblasts. J Neural Transm Suppl. 2003. 67:235–242.
Article14. Gazzolo D, Bruschettini M, Corvino V, Lituania M, Sarli R, Bruschettini P, Michetti F. Amniotic fluid levels of S100B protein in normal and trisomy-21 foetuses. Clin Chim Acta. 2003. 330:131–133.
Article15. Heizmann CW. Ca2+-binding S100 proteins in the central nervous system. Neurochem Res. 1999. 24:1097–1100.16. Raijmakers MT, Steegers EA, Peters WH. Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues. Hum Reprod. 2001. 16:2445–2450.
Article17. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994. 300(Pt 1):271–276.
Article18. Engidawork E, Baiic N, Fountoulakis M, Dierssen M, Greber-Platzer S, Lubec G. Beta-amyloid precursor protein, ETS-2 and collagen alpha 1 (VI) chain precursor, encoded on chromosome 21, are not overexpressed in fetal Down syndrome: further evidence against gene dosage effect. J Neural Transm Suppl. 2001. 61:335–346.19. Davies GE, Howard CM, Farrer MJ, Coleman MM, Bennett LB, Cullen LM, Wyse RK, Burn J, Williamson R, Kessling AM. Genetic variation in the COL6A1 region is associated with congenital heart defects in trisomy 21 (Down's syndrome). Ann Hum Genet. 1995. 59(Pt 3):253–269.
Article20. Loftis MJ, Sexton D, Carver W. Effects of collagen density on cardiac fibroblast behavior and gene expression. J Cell Physiol. 2003. 196:504–511.
Article21. Anlar B, Atilla P, Cakar AN, Kose MF, Beksac MS, Dagdeviren A, Akcoren Z. Expression of adhesion and extracellular matrix molecules in the developing human brain. J Child Neurol. 2002. 17:707–713.
Article22. Olsen BR. The roles of collagen genes in skeletal development and morphogenesis. Experientia. 1995. 51:194–195.
Article23. Superti-Furga A, Gugler E, Gitzelmann R, Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988. 263:6226–6232.
Article24. Sugiura T, Takamatsu H, Kudo A, Amann E. Expression and characterization of murine osteoblast-specific factor 2 (OSF-2) in a baculovirus expression system. Protein Expr Purif. 1995. 6:305–311.
Article25. Engidawork E, Lubec G. Protein expression in Down syndrome brain. Amino Acids. 2001. 21:331–361.
Article26. Gulesserian T, Engidawork E, Yoo BC, Cairns N, Lubec G. Alteration of caspases and other apoptosis regulatory proteins in Down syndrome. J Neural Transm Suppl. 2001. 61:163–179.
Article27. Yasuda M, Chinnadurai G. Functional identification of the apoptosis effector BH3 domain in cellular protein BNIP1. Oncogene. 2000. 19:2363–2367.
Article28. Cheon MS, Shim KS, Kim SH, Hara A, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: Challenging the gene dosage effect hypothesis (Part IV). Amino Acids. 2003. 25:41–47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basic Concept of Gene Microarray
- Acetaminophen Induced Cytotoxicity and Altered Gene Expression in Cultured Cardiomyocytes of H9C2 Cells
- Profiling of Differentially Expressed Genes in Human Polymorphonuclear Leukocyte on Human Amniotic Membrane
- Gene Expression Analysis of Murine Primary Microglia Stimulated with LPS using Microarray
- Microarray Analysis of Oxygen-Glucose-Deprivation Induced Gene Expression in Cultured Astrocytes