Yonsei Med J.
2007 Apr;48(2):308-316. 10.3349/ymj.2007.48.2.308.
Induction of PPAR Gamma mRNA and Protein Expression by Rosiglitazone in Chronic Cyclosporine Nephropathy in the Rat
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea. yangch@catholic.ac.kr
- 2Department of Internal Medicine, The Affiliated Hospital, YanBian University Medical College, YanJi 133000, JiLin, PR China.
- 3Division of Nephrology, Endocrinology and Vascular Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan.
- 4Cell Death Research Center, Department of Anatomy, The Catholic University of Korea, Seoul, Korea.
- KMID: 1779536
- DOI: http://doi.org/10.3349/ymj.2007.48.2.308
Abstract
- PURPOSE
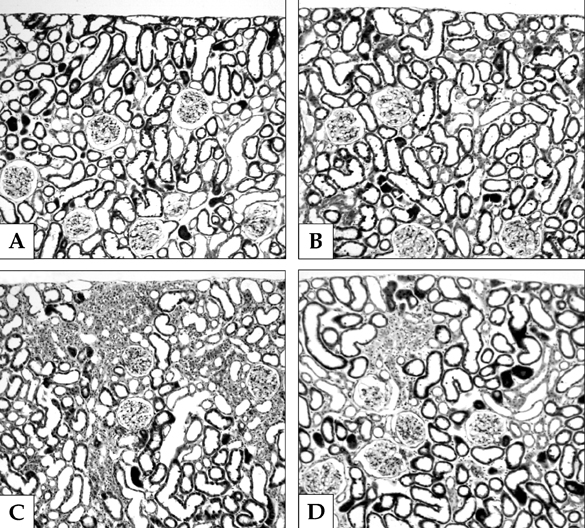
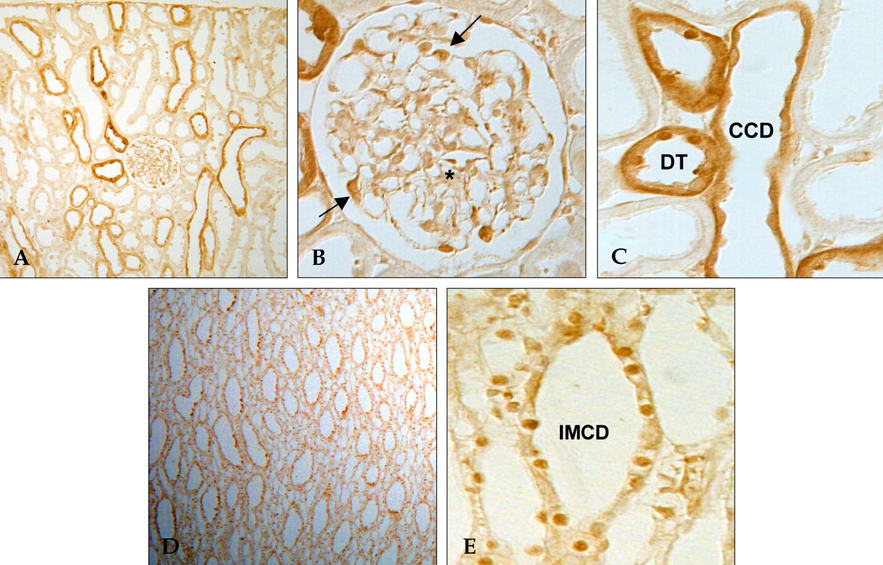
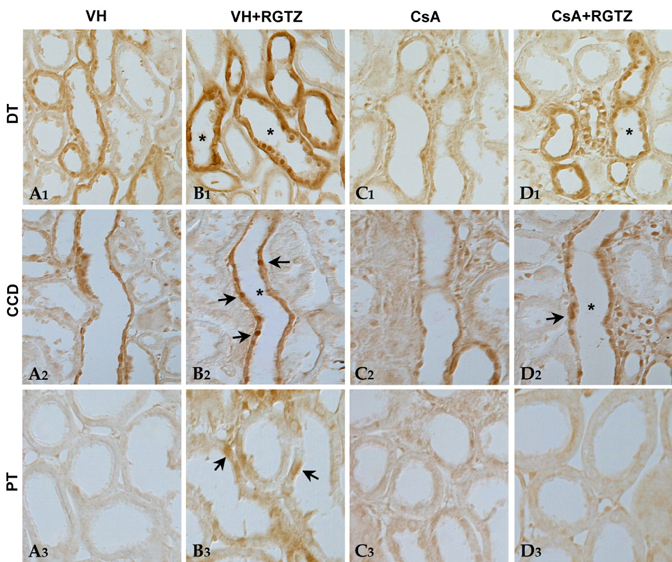
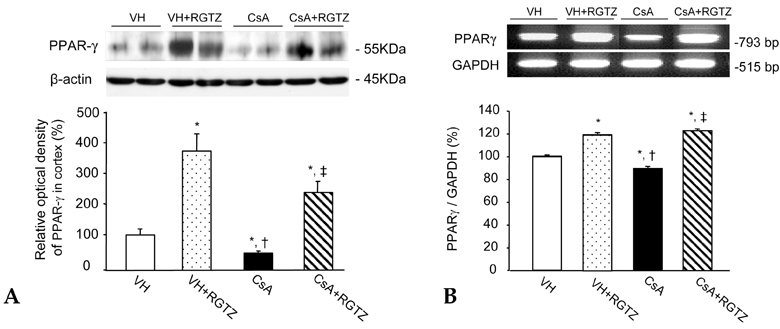
We recently reported that rosiglitazone (RGTZ), a peroxisome proliferator-activated receptor gamma (PPARgamma) agonist, has a protective effect against cyclosporine (CsA)- induced renal injury. Here we report the effect of RGTZ on peroxisome proliferator-activated receptor gamma (PPARgamma) expression in an experimental model of chronic cyclosporine (CsA) nephropathy. MATERIALS AND METHODS: Chronic CsA nephropathy was induced in Sprague-Dawley rats by administering CsA (15mg/kg per day) for 28 days, and control rats were treated with vehicle (VH group, olive oil 1mL/kg per day) for 28 days. RGTZ (3mg/kg) was concurrently administered via gavage to the CsA and VH groups. Expression of PPARgamma mRNA and protein was evaluated with RT-PCR, immunohistochemistry, and immunoblotting. RESULTS: PPARgamma mRNA expression was similar to the level of PPARgamma protein constitutively expressed in the kidneys of the VH treated rats, with expression in the glomerular epithelial, distal tubular cells, and collecting tubular cells. PPARgamma protein expression in CsA-treated rat kidneys was significantly less than in the VH group. However, concomitant administration of RGTZ restored PPARgamma protein expression in the kidneys of the CsA- reated rats. CONCLUSION: Exogenous administration of RGTZ treatment upregulates PPARgamma expression and that this mechanism may play a role in protecting against CsA-induced renal injury.
MeSH Terms
-
Transcription, Genetic/*drug effects
Thiazolidinediones/*pharmacology
Rats, Sprague-Dawley
Rats
RNA, Messenger/*genetics
Protein Biosynthesis/*drug effects
PPAR gamma/*genetics
Male
Kidney Diseases/genetics/pathology/*prevention & control
Gene Expression Regulation/*drug effects
Disease Models, Animal
Cyclosporine/*toxicity
Animals
Figure
Reference
-
1. Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney Int. 1996. 50:1089–1100.2. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984. 311:699–705.3. Campistol JM, Sacks SH. Mechanisms of nephrotoxicity. Transplantation. 2000. 69:12 Suppl. SS5–SS10.4. Pichler RH, Franceschini N, Young BA, Hugo C, Andoh TF, Burdmann EA, et al. Pathogenesis of cyclosporine nephropathy: roles of angiotensin II and osteopontin. J Am Soc Nephrol. 1995. 6:1186–1196.5. Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001. 60:14–30.6. Sato K, Sugawara A, Kudo M, Uruno A, Ito S, Takeuchi K. Expression of peroxisome proliferator-activated receptor isoform proteins in the rat kidney. Hypertens Res. 2004. 27:417–425.7. Buckingham RE, Al-Barazanji KA, Toseland CD, Slaughter M, Connor SC, West A, et al. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998. 47:1326–1334.8. Yue Tl TL, Chen J, Bao W, Narayanan PK, Bril A, Jiang W, et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2001. 104:2588–2594.9. Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002. 161:1027–1040.10. Chung BH, Li C, Sun BK, Lim SW, Ahn KO, Yang JH, et al. Rosiglitazone protects against cyclosporine-induced pancreatic and renal injury in rats. Am J Transplant. 2005. 5:1856–1867.11. Sugawara A, Yen PM, Qi Y, Lechan RM, Chin WW. Isoform-specific retinoid-X receptor (RXR) antibodies detect differential expression of RXR proteins in the pituitary gland. Endocrinology. 1995. 136:1766–1774.12. Lim SW, Li C, Sun BK, Han KH, Kim WY, Oh YW, et al. Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat kidney. Am J Physiol Renal Physiol. 2004. 287:F139–F151.13. Yang CW, Ahn HJ, Kim WY, Li C, Jung JY, Yoon SA, et al. Synergistic effects of mycophenolate mofetil and losartan in a model of chronic cyclosporine nephropathy. Transplantation. 2003. 75:309–315.14. Yang T, Michele DE, Park J, Smart AM, Lin Z, Brosius FC 3rd, et al. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. Am J Physiol. 1999. 277:F966–F973.15. Healy E, Dempsey M, Lally C, Ryan MP. Apoptosis and necrosis: mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 1998. 54:1955–1966.16. Kim SI, Song HY, Hwang JH, Chong DL, Lee HY, Han DS, et al. Cyclosporine nephrotoxicity: the mechanisms of cell injury by cyclosporine A in renal proximal tubular cells. Transplant Proc. 2000. 32:1621–1622.17. Hortelano S, Castilla M, Torres AM, Tejedor A, Bosca L. Potentiation by nitric oxide of cyclosporin A and FK506-induced apoptosis in renal proximal tubule cells. J Am Soc Nephrol. 2000. 11:2315–2323.18. Slattery C, Campbell E, McMorrow T, Ryan MP. Cyclosporine A-induced renal fibrosis: a role for epithelial-mesenchymal transition. Am J Pathol. 2005. 167:395–407.19. McMorrow T, Gaffney MM, Slattery C, Campbell E, Ryan MP. Cyclosporine A induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2005. 20:2215–2225.20. Bone JM, Amara AB, Shenkin A, Hammad A, Sells RA, Alexander JL, et al. Calcineurin inhibitors and proximal renal tubular injury in renal transplant patients with proteinuria and chronic allograft nephropathy. Transplantation. 2005. 79:119–122.21. Yang CW, Ahn HJ, Kim WY, Shin MJ, Kim SK, Park JH, et al. Influence of the renin-angiotensin system on epidermal growth factor expression in normal and cyclosporine-treated rat kidney. Kidney Int. 2001. 60:847–857.22. Sugawara A, Uruno A, Kudo M, Ikeda Y, Sato K, Taniyama Y, et al. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-gamma via an interaction with Sp1 in vascular smooth muscle cells. J Biol Chem. 2002. 277:9676–9683.23. Darlametsos IE, Papanikolaou EN, Varonos DD. Effect of nifedipine in cyclosporine-induced nephrotoxicity in rats: roles of the thromboxane and endothelin systems. Prostaglandins Leukot Essent Fatty Acids. 2000. 63:263–269.24. Darlametsos IE, Varonos DD. Role of prostanoids and endothelins in the prevention of cyclosporine-induced nephrotoxicity. Prostaglandins Leukot Essent Fatty Acids. 2001. 64:231–239.25. Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005. 11:861–866.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression of RANKL and OPG by the PPAR gamma Agonist Rosiglitazone in Osteoblasts
- Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter
- The PPARgamma Agonist Rosiglitazone Inhibits Glioma Cell Proliferation and Migration in vitro and Glioma Tumor Growth in vivo
- Expression of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma in the lung tissue of obese mice and the effect of rosiglitazone on proinflammatory cytokine expressions in the lung tissue
- The Effect of Chlamydia pneumoniae on the Expression of Peroxisome Proliferator-Activated Receptor-gamma in Vascular Smooth Muscle Cells