Yonsei Med J.
2008 Apr;49(2):230-236. 10.3349/ymj.2008.49.2.230.
The Effect of Chlamydia pneumoniae on the Expression of Peroxisome Proliferator-Activated Receptor-gamma in Vascular Smooth Muscle Cells
- Affiliations
-
- 1Department of Cardiothoracic Surgery, Catholic University College of Medicine, Seoul, Korea. kiyuk@catholic.ac.kr
- 2Department of Internal Medicine, Catholic University College of Medicine, Seoul, Korea.
- KMID: 1084495
- DOI: http://doi.org/10.3349/ymj.2008.49.2.230
Abstract
- PURPOSE
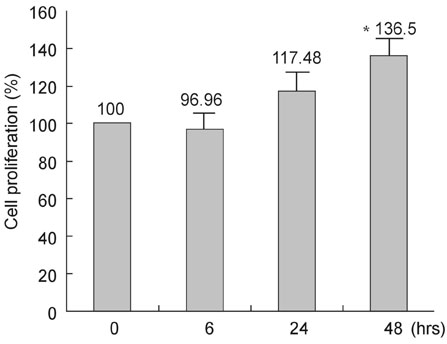
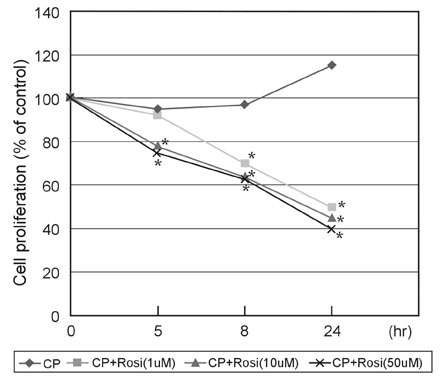
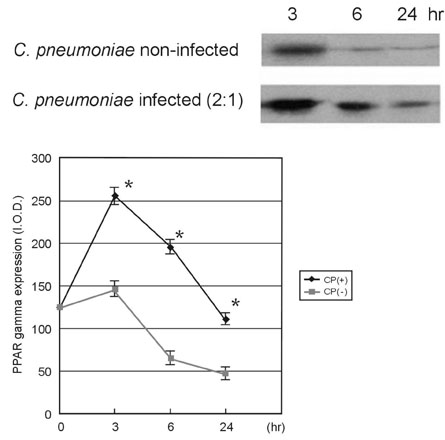
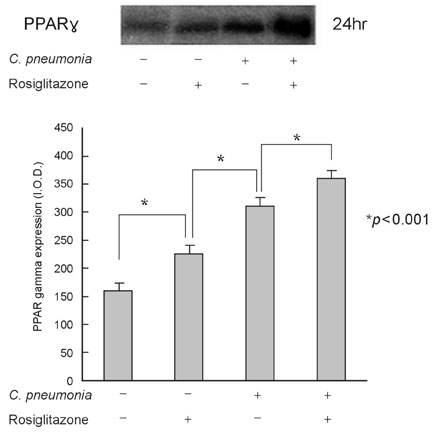
This study was designed to investigate the change of peroxisome proliferator-activated receptor gamma (PPARgamma) after the infection of the human coronary artery smooth muscle cells (HCSMCs) with Chlamydia pneumoniae (C. pneumoniae) and the effect of PPARgamma agonist on the expression of PPARgamma of C. pneumoniae-infected HCSMCs. MATERIALS AND METHODS: To determine the effect of PPARgamma agonist on the proliferation of C. pneumoniae-infected HCSMCs, rosiglitazone at various concentrations was applied 1 hour before inoculation of HCSMCs. RESULTS: The expression of PPARgamma mRNA in HCSMCs increased from 3 hours after C. pneumoniae infection and reached that of noninfected HCSMCs at 24 hours (p < 0.05). The expression of PPARgamma protein in HCSMCs also increased from 3 hours after C. pneumoniae and persisted until 24 hours as compared with that of noninfected HCSMCs (p < 0.05). The pretreatment of HCSMCs with rosiglitazone followed by the infection with C. pneumoniae augmented the expression of PPARgamma mRNA and protein (p < 0.05) and decreased cell proliferation. CONCLUSION: Our results showed that the expression of PPARgamma increases in response to C. pneumoniae infection and rosiglitazone further augmented the expression of PPARgamma. It is suggested that rosiglitazone could ameliorate the chronic inflammation in the vessel wall induced by C. pneumoniae by augmenting PPARgamma expression.
Keyword
MeSH Terms
-
Blotting, Western
Cell Line
Cell Proliferation/drug effects
Chlamydophila pneumoniae/growth & development/*physiology
Gene Expression Regulation/drug effects
Humans
Muscle, Smooth, Vascular/cytology/drug effects/metabolism
Myocytes, Smooth Muscle/drug effects/*metabolism/microbiology
PPAR gamma/genetics/*metabolism
RNA, Messenger/genetics/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Thiazolidinediones/pharmacology
Figure
Reference
-
1. Leinonen M, Saikku P. Evidence for infectious agents in cardiovascular disease and atherosclerosis. Lancet Infect Dis. 2002. 2:11–17.
Article2. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Chlamydia pneumoniae IgG titers and coronary heart disease: prospective study and meta-analysis. BMJ. 2000. 321:208–213.
Article3. Gibbs RG, Carey N, Davies AH. Chlamydia pneumoniae and vascular disease. Br J Surg. 1998. 85:1191–1197.4. Gaydos CA, Summersgill JT, Sahney NN, Ramirez JA, Quinn TC. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996. 64:1614–1620.
Article5. Lin TM, Campbell LA, Rosenfeld ME, Kuo CC. Monocyte-endothelial cell coculture enhances infection of endothelial cells with Chlamydia pneumoniae. J Infect Dis. 2000. 181:1096–1100.
Article6. Moazed TC, Kuo CC, Grayston JT, Campbell LA. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998. 177:1322–1325.
Article7. Da Costa CU, Wantia N, Kirschning CJ, Busch DH, Rodriguez N, Wagner H, et al. Heat shock protein 60 from Chlamydia pneumoniae elicits an unusual set of inflammatory responses via Toll-like receptor 2 and 4 in vivo. Eur J Immunol. 2004. 34:2874–2884.
Article8. Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, et al. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-Kappa B and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation. 1999. 100:1369–1373.
Article9. Fryer RH, Schwobe EP, Woods ML, Rodgers GM. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Investig Med. 1997. 45:168–174.10. Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B. The role of PPARs in atherosclerosis. Trends Mol Med. 2002. 8:422–430.
Article11. Hsueh WA, Jackson S, Law RE. Control of vascular cell proliferation and migration by PPAR-gamma: a new approach to the macrovascular complications of diabetes. Diabetes Care. 2001. 24:392–397.12. Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPAR gamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma (PPAR gamma) expression and reduction of MMP-9 activity through PPAR gamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998. 153:17–23.13. Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, et al. Expression of the peroxisome proliferator-activated receptor gamma (PPAR gamma) in human atherosclerosis and regulation in macrophages by colon stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998. 95:7614–7619.
Article14. Murao K, Imachi H, Momoi A, Sayo Y, Hosokawa H, Sato M, et al. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999. 454:27–30.
Article15. Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor gamma activators. Circulation. 2000. 101:235–238.
Article16. Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998. 83:1097–1103.
Article17. Leininger MT, Portocarrero CP, Houseknecht KL. Peroxisome proliferator-activated receptor gamma 1 expression in porcine white blood cells: dynamic regulation with acute endotoxemia. Biochem Biophys Res Commun. 1999. 263:749–753.
Article18. Hill MR, Young MD, McCurdy CM, Gimble JM. Decreased expression of murine PPAR gamma in adipose tissue during endotoxemia. Endocrinology. 1997. 138:3073–3076.
Article19. Liu L, Hu H, Ji H, Murdin AD, Pierce GN, Zhong G. Chlamydia pneumoniae infection significantly exacerbates aortic atherosclerosis in an LDLR-/- mouse model within six months. Mol Cell Biochem. 2000. 215:123–128.20. Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999. 180:238–241.
Article21. Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, et al. Expression and function of PPAR gamma in rat and human vascular smooth muscle cells. Circulation. 2000. 101:1311–1318.
Article22. Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1 →S transition in vascular smooth muscle cells. J Biol Chem. 2000. 275:22435–22441.
Article23. Goetze S, Xi XP, Kawano H, Gotlibowski T, Fleck E, Hsueh WA, et al. PPAR gamma-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1999. 33:798–806.
Article24. Konturek PC, Kania J, Konturek JW, Nikiforuk A, Konturek SJ, Hahn EG. H. pylori infection, atrophic gastritis, cytokines, gastrin, COX-2, PPAR gamma and impaired apoptosis in gastric carcinogenesis. Med Sci Monit. 2003. 9:SR53–SR66.25. Huang B, Dong Y, Mai W, Li Y. Effect of Chlamydia pneumoniae infection and hyperlipidaemia on the expression of PPAR gamma, P50 and c-Fos in aortic endothelial cells in C57bL/6J mice. Acta Cardiol. 2005. 60:43–49.
Article26. Casteleijn E, Kuiper J, Van Rooij HC, Kamps JA, Koster JF, Van Berkel TJ. Endotoxin stimulates glycogenolysis in the liver by means of intercellular communication. J Biol Chem. 1988. 263:6953–6955.
Article27. McGuire JC, Richard KA, Sun FF, Tracey DE. Production of prostaglandin D2 by murine macrophage cell lines. Prostaglandins. 1985. 30:949–967.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcriptional Regulation of Insulin and CXCL10 Gene by Peroxisome Proliferator Activated Receptor gamma Coactivator-1alpha
- The Effects of Troglitazone on Vascular Smooth Muscle Cell Proliferation
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Peroxisome Proliferator Activated Receptor-delta (PPAR-delta)
- Transporters and Nuclear Hormone Receptors associated with Cholesterol Metabolism in Gallbladder Epithelial Cells