Korean Circ J.
2008 Sep;38(9):446-454. 10.4070/kcj.2008.38.9.446.
Therapeutic Potential of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Ischemic Myocardium
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Gwangju, Korea.
- 2Cardiovascular Research Institute of Chonnam National University, Gwangju, Korea.
- 3JB Stem Cell Institute, Gwangju, Korea.
- 4Department of Obstetrics & Gynecology, Chosun University Hospital, Gwangju, Korea.
- KMID: 1776344
- DOI: http://doi.org/10.4070/kcj.2008.38.9.446
Abstract
- BACKGROUND AND OBJECTIVES
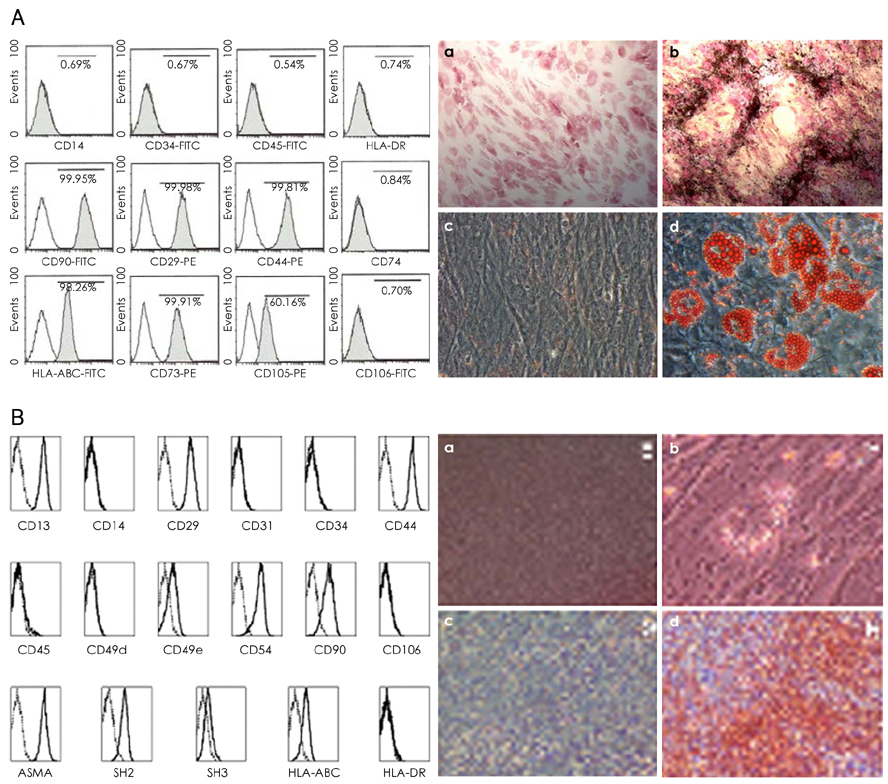
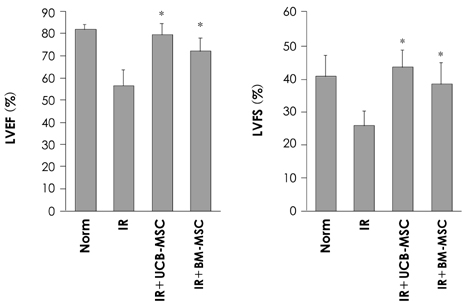
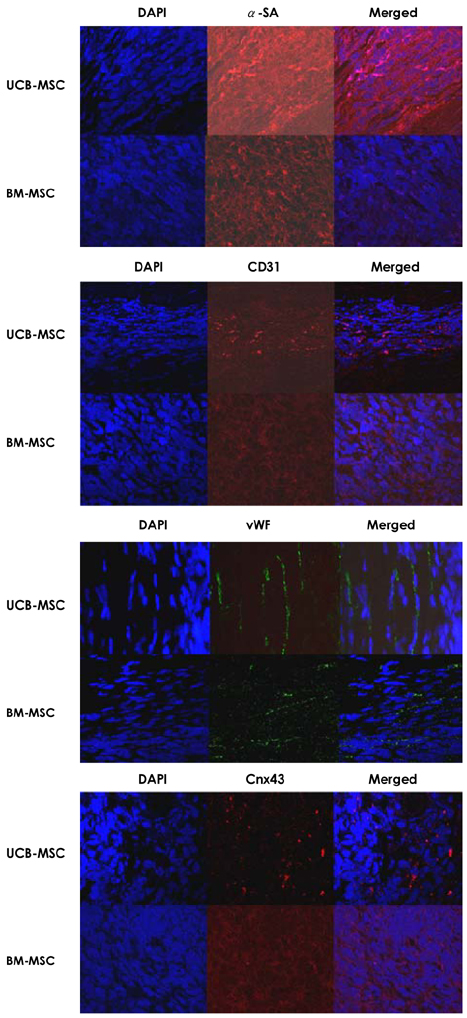
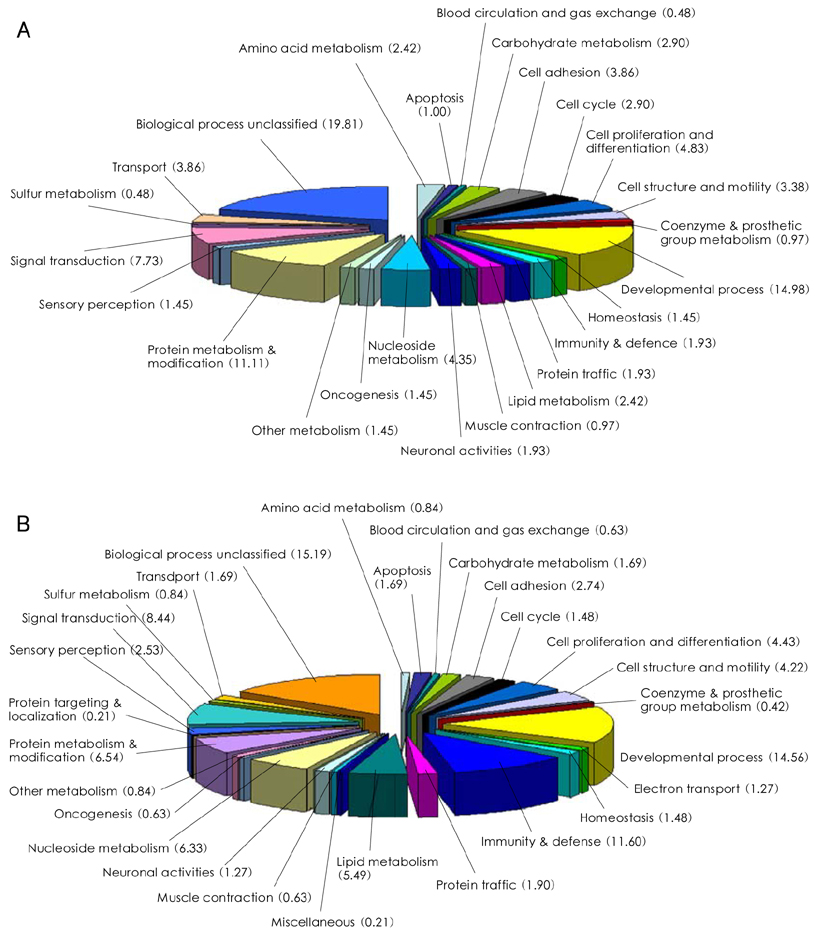
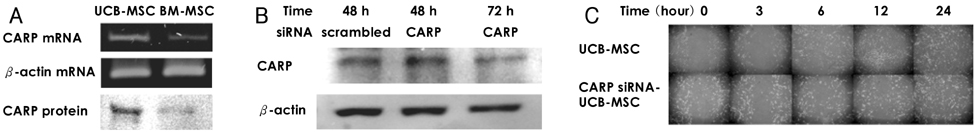
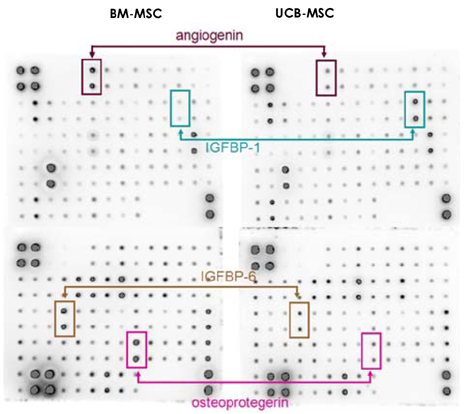
We designed this study to determine the therapeutic potentials of umbilical cord blood (UCB)-mesenchymal stem cells (MSCs), as compared with bone marrow (BM)-MSCs. MATERIALS AND METHODS: MSCs were isolated from UCB and BM. For the in vivo study, myocardial infarction was induced by ligation of the left anterior descending coronary artery (LAD) in rats for 30 min, and this was followed by release; the MSCs were then injected into a designated point around the infarcted area. Echocardiographs were performed two weeks after surgery. For the in vitro study, a cDNA microarray and cytokine array were performed to compare the MSCs from UCB and from BM. Cell migration was assessed by a wound scratch assay, and the level of cardiac ankyrin repeat protein (CARP) was determined by reverse transcriptase-polymer chain reaction (RT-PCR) or Western blot analysis. RESULTS: For the echocardiograph findings, the fractional shortening (FS) was 43.9% in the UCB-MSCs group and it was 38.6% in the BM-MSC group. The ejection fraction (EF) was 79.8% in the UCB-MSC group and it was 72.4% in the BM-MSC group (control FS: 26.2% and the control EF: 56.6%). CARP was one of the highly expressed genes in the UCB-MSCs on the cDNA microarray. The mRNA and the expressed level of CARP protein in the UCB-MSCs were higher than those in the BM-MSCs. The cell migration of the CARP small interfering ribonucleic acid (siRNA) transfected UCB-MSCs was delayed compared to that of the normal UCB-MSCs (p<0.05) CONCLUSION: Our study directly compared the two types of MSCs from UCB and BM, and we suggest that the CARP molecule might be responsible for the motility of UCB-MSCs.
MeSH Terms
Figure
Reference
-
1. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.2. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.3. Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005. 102:11474–11479.4. Piao H, Youn TJ, Kwon JS, et al. Cellular cardiomyoplsty using bone marrow derived mesenchymal stem cells transplantation in post myocardial infarction heart failure. Korean Circ J. 2004. 34:1113–1121.5. Lim SY, Jeong MH, Ahn YK, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Korean Circ J. 2005. 35:734–741.6. Zhang H, Fazel S, Tian H, et al. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005. 289:H2089–H2096.7. Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004. 22:625–634.8. Moon MH, Kim SY, Kim YJ, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006. 17:279–290.9. Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005. 79:528–535.10. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006. 12:459–465.11. Tsai MS, Hwang SM, Chen KD, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. 2007. 25:2511–2523.12. Han XJ, Chae JK, Lee MJ, You KR, Lee BH, Kim DG. Involvement of GADD153 and cardiac ankyrin repeat protein in hypoxia-induced apoptosis of H9c2 cells. J Biol Chem. 2005. 280:23122–23129.13. Nakada C, Oka A, Nonaka I, et al. Cardiac ankyrin repeat protein is preferentially induced in atrophic myofibers of congenital myopathy and spinal muscular atrophy. Pathol Int. 2003. 53:653–658.14. Shi Y, Reitmaier B, Regenbogen J, et al. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol. 2005. 166:303–312.15. Chen CL, Lin JL, Lai LP, Pan CH, Huang SK, Lin CS. Altered expression of FHL1, CARP, TSC-22 and P311 provide insights into complex transcriptional regulation in pacing-induced atrial fibrillation. Biochim Biophys Acta. 2007. 1772:317–329.16. Qi CM, Ma GS, Liu NF, et al. Identification and differentiation of magnetically labeled mesenchymal stem cells in vivo in swines with myocardial infarction. Int J Cardiol. 2007. [Epub ahead of print].17. Numaguchi Y, Sone T, Okumura K, et al. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006. 114:1 suppl. I114–I119.18. Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005. 80:229–236.19. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.20. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.21. Pagani FD, DerSimonian H, Zawadzka A, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans: histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003. 41:879–888.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation