Korean J Physiol Pharmacol.
2012 Jun;16(3):167-174. 10.4196/kjpp.2012.16.3.167.
Generation of CD2+CD8+ NK Cells from c-kit+ Bone Marrow Cells in Porcine
- Affiliations
-
- 1Department of Physiology and Biosafety Research Institute, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea. jhkim1@chonbuk.ac.kr
- 2Department of Pathology, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea.
- 3Department of Laboratroy Animal Medicine, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea.
- 4Department of Parasitology, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea.
- 5Department of Veterinary Physiology, College of Veterinary Medicine, Seoul National University, Seoul 151-741, Korea.
- 6Department of Clinical Neurosciences, Neurology Unit, Addenbrooke's Hospital, University of Cambridge, Cambridge CB2 0QQ, UK.
- KMID: 1768008
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.167
Abstract
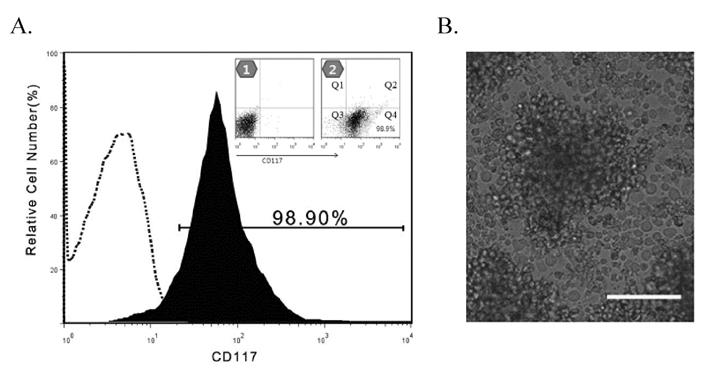
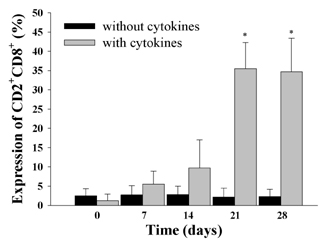
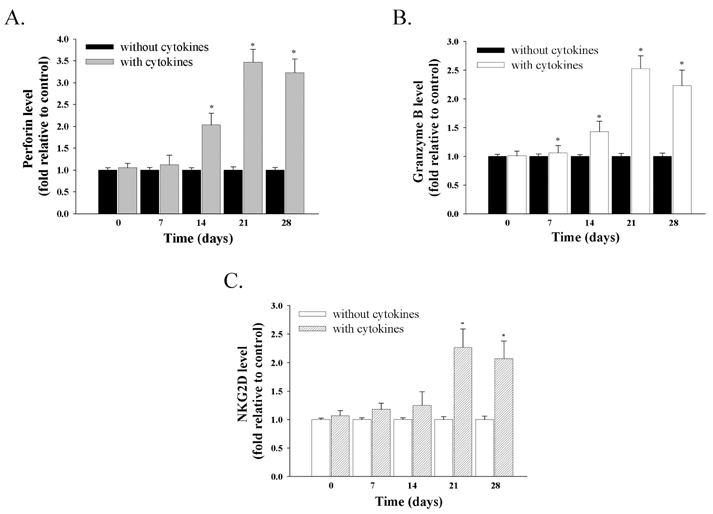
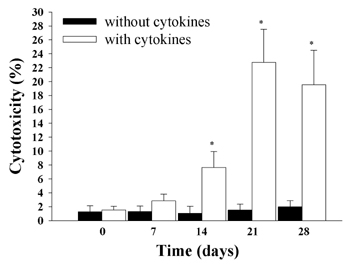
- Natural killer (NK) cells provide one of the initial barriers of cellular host defense against pathogens, in particular intracellular pathogens. Because bone marrow-derived hematopoietic stem cells (HSCs), lymphoid protenitors, can give rise to NK cells, NK ontogeny has been considered to be exclusively lymphoid. Here, we show that porcine c-kit+ bone marrow cells (c-kit+ BM cells) develop into NK cells in vitro in the presence of various cytokines [interleukin (IL)-2, IL-7, IL-15, IL-21, stem cell factor (SCF), and fms-like tyrosine kinase-3 ligand (FLT3L)]. Adding hydrocortisone (HDC) and stromal cells greatly increases the frequency of c-kit+ BM cells that give rise to CD2+CD8+ NK cells. Also, intracellular levels of perforin, granzyme B, and NKG2D were determined by RT-PCR and western blotting analysis. It was found that of perforin, granzyme B, and NKG2D levels significantly were increased in cytokine-stimulated c-kit+ BM cells than those of controls. And, we compared the ability of the cytotoxicity of CD2+CD8+ NK cells differentiated by cytokines from c-kit+ BM cells against K562 target cells for 28 days. Cytokines-induced NK cells as effector cells were incubated with K562 cells as target in a ratio of 100:1 for 4 h once a week. In results, CD2+CD8+ NK cells induced by cytokines and stromal cells showed a significantly increased cytotoxicity 21 days later. Whereas, our results indicated that c-kit+ BM cells not pretreated with cytokines have lower levels of cytotoxicity. Taken together, this study suggests that cytokines-induced NK cells from porcine c-kit+ BM cells may be used as adoptive transfer therapy if the known obstacles to xenografting (e.g. immune and non-immune problems) were overcome in the future.
MeSH Terms
-
Adoptive Transfer
Blotting, Western
Bone Marrow
Bone Marrow Cells
Cytokines
Granzymes
Hematopoietic Stem Cells
Hydrocortisone
Interleukin-15
Interleukin-7
Interleukins
K562 Cells
Killer Cells, Natural
Perforin
Stem Cell Factor
Stromal Cells
Transplantation, Heterologous
Tyrosine
Cytokines
Granzymes
Hydrocortisone
Interleukin-15
Interleukin-7
Interleukins
Perforin
Stem Cell Factor
Tyrosine
Figure
Reference
-
1. Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994. 43:185–191.2. Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002. 53:133–147.3. de Vries-van der Zwan A, van der Pol MA, Besseling AC, de Waal LP, Boog CJ. Haematopoietic stem cells can induce specific skin graft acceptance across full MHC barriers. Bone Marrow Transplant. 1998. 22:91–98.4. Abe M, Qi J, Sykes M, Yang YG. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood. 2002. 99:3823–3829.5. Kawashima I, Zanjani ED, Almaida-Porada G, Flake AW, Zeng H, Ogawa M. CD34+ human marrow cells that express low levels of Kit protein are enriched for long-term marrow-engrafting cells. Blood. 1996. 87:4136–4142.6. Dalakas E, Newsome PN, Harrison DJ, Plevris JN. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005. 19:1225–1231.7. Ortaldo JR, Herberman RB. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984. 2:359–394.8. Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997. 91:661–672.9. Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003. 3:413–425.10. Perez SA, Sotiropoulou PA, Gkika DG, Mahaira LG, Niarchos DK, Gritzapis AD, Kavalakis YG, Antsaklis AI, Baxevanis CN, Papamichail M. A novel myeloid-like NK cell progenitor in human umbilical cord blood. Blood. 2003. 101:3444–3450.11. Márquez C, Trigueros C, Franco JM, Ramiro AR, Carrasco YR, López-Botet M, Toribio ML. Identification of a common developmental pathway for thymic natural killer cells and dendritic cells. Blood. 1998. 91:2760–2771.12. Lotzová E, Savary CA. Human natural killer cell development from bone marrow progenitors: analysis of phenotype, cytotoxicity and growth. Nat Immun. 1993. 12:209–217.13. Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994. 83:2594–2601.14. Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, Caligiuri MA. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood. 1998. 92:3647–3657.15. Yoon SR, Chung JW, Choi I. Development of natural killer cells from hematopoietic stem cells. Mol Cells. 2007. 24:1–8.16. Mrózek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996. 87:2632–2640.17. Williams NS, Klem J, Puzanov IJ, Sivakumar PV, Bennett M, Kumar V. Differentiation of NK1.1+, Ly49+ NK cells from flt3+ multipotent marrow progenitor cells. J Immunol. 1999. 163:2648–2656.18. Whiteside TL, Vujanovic NL, Herberman RB. Natural killer cells and tumor therapy. Curr Top Microbiol Immunol. 1998. 230:221–244.19. Grzywacz B, Kataria N, Kataria N, Blazar BR, Miller JS, Verneris MR. Natural killer-cell differentiation by myeloid progenitors. Blood. 2011. 117:3548–3558.20. Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999. 162:6658–6662.21. Pintaric M, Gerner W, Saalmüller A. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-gamma production of porcine natural killer cells. Vet Immunol Immunopathol. 2008. 121:68–82.22. Sotiriadis J, Shin SC, Yim D, Sieber D, Kim YB. Thomsen-Friedenreich (T) antigen expression increases sensitivity of natural killer cell lysis of cancer cells. Int J Cancer. 2004. 111:388–397.23. Layton DS, Strom AD, O'Neil TE, Broadway MM, Stephenson GL, Morris KR, Muralitharan M, Sandrin MS, Ierino FL, Bean AG. Development of an anti-porcine CD34 monoclonal antibody that identifies hematopoietic stem cells. Exp Hematol. 2007. 35:171–178.24. Le Guern AC, Giovino MA, Abe M, Theodore PR, Qi J, Down JD, Sachs DH, Sykes M, Yang YG. Stem cell activity of porcine c-kit+ hematopoietic cells. Exp Hematol. 2003. 31:833–840.25. Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, Miller JS, Verneris MR. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006. 108:3824–3833.26. Shen T, Lee JH, Park MH, Lee YG, Rho HS, Kwak YS, Rhee MH, Park YC, Cho JY. Ginsenoside Rp1, a Ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKb-mediated NF-kB pathway in HEK293 Cells. J Ginseng Res. 2011. 35:200–208.27. Yim D, Jie HB, Sotiriadis J, Kim YS, Kim KS, Rothschild MF, Lanier LL, Kim YB. Molecular cloning and characterization of pig immunoreceptor DAP10 and NKG2D. Immunogenetics. 2001. 53:243–249.28. Seo EY, Kim WK. Red Ginseng extract reduced metastasis of colon cancer cells in vitro and in vivo. J Ginseng Res. 2011. 35:315–324.29. Yoo DS, Rho HS, Lee YG, Yeom MH, Kim DH, Lee SJ, Hong SY, Lee JH, Cho JY. Ginsenoside F1 modulates cellular responses of skin melanoma cells. J Ginseng Res. 2011. 35:86–91.30. Wrona D, Sukiennik L, Jurkowski MK, Jurkowlaniec E, Glac W, Tokarski J. Effects of amphetamine on NK-related cytotoxicity in rats differing in locomotor reactivity and social position. Brain Behav Immun. 2005. 19:69–77.31. Toka FN, Nfon CK, Dawson H, Estes DM, Golde WT. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J Interferon Cytokine Res. 2009. 29:179–192.32. Denyer MS, Wileman TE, Stirling CM, Zuber B, Takamatsu HH. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol. 2006. 110:279–292.33. Zhang B, Zhang J, Tian Z. Comparison in the effects of IL-2, IL-12, IL-15 and IFNalpha on gene regulation of granzymes of human NK cell line NK-92. Int Immunopharmacol. 2008. 8:989–996.34. Kang SG, Jeun SS, Lim JY, Yoo DS, Huh PW, Cho KS, Kim DS, Shin HJ, Kim JH, Kim MC, Kang JK. Cytotoxicity of rat marrow stromal cells against malignant glioma cells. Childs Nerv Syst. 2005. 21:528–538.35. Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006. 214:56–72.36. Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006. 17:259–280.37. Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994. 180:1395–1403.38. Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005. 174:1213–1221.39. Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Münz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004. 172:1455–1462.40. Briard D, Brouty-Boyé D, Azzarone B, Jasmin C. Fibroblasts from human spleen regulate NK cell differentiation from blood CD34(+) progenitors via cell surface IL-15. J Immunol. 2002. 168:4326–4332.41. Boysen P, Storset AK. Bovine natural killer cells. Vet Immunol Immunopathol. 2009. 130:163–177.42. Gerner W, Käser T, Saalmüller A. Porcine T lymphocytes and NK cells--an update. Dev Comp Immunol. 2009. 33:310–320.43. Toka FN, Nfon CK, Dawson H, Estes DM, Golde WT. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J Interferon Cytokine Res. 2009. 29:179–192.44. Pintaric M, Gerner W, Saalmüller A. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-gamma production of porcine natural killer cells. Vet Immunol Immunopathol. 2008. 121:68–82.45. Setcavage TM, Kim YB. Variability of the immunological state of germfree colostrum-deprived Minnesota miniature piglets. Infect Immun. 1976. 13:600–607.46. Yim D, Jie HB, Lanier LL, Kim YB. Molecular cloning, gene structure, and expression pattern of pig immunoreceptor DAP12. Immunogenetics. 2000. 51:436–442.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation and Characterization of Alloenic Radiation Bone Marrow Chimera
- Reconstitution of Bone Marrow after Allogeneic and Autologous Bone Marrow Transplantation
- The effect of interleukin 2 on the induction Of Nk 1.1 expression in CD8+ and CD4-CD8-T Cell
- Human CD8+ T-Cell Populations That Express Natural Killer Receptors
- Positive Selection of Transgenic CD4 T Cells in the Restricted Peptide Environment