J Bacteriol Virol.
2006 Mar;36(1):1-10. 10.4167/jbv.2006.36.1.1.
Effective Microorgainsm (EM) Fermentation Extract Attenuates Airway Hyperreactivity and Lung Inflammation In A Mouse Model of Asthma
- Affiliations
-
- 1Department of Biological Science, School of Science and Technology, Jeonju University, Jeonju 560-759, Korea. sangyun@jj.ac.kr
- 2The Center for EM Research and Development, Jeonju University, Jeonju 560-759, Korea.
- KMID: 1719019
- DOI: http://doi.org/10.4167/jbv.2006.36.1.1
Abstract
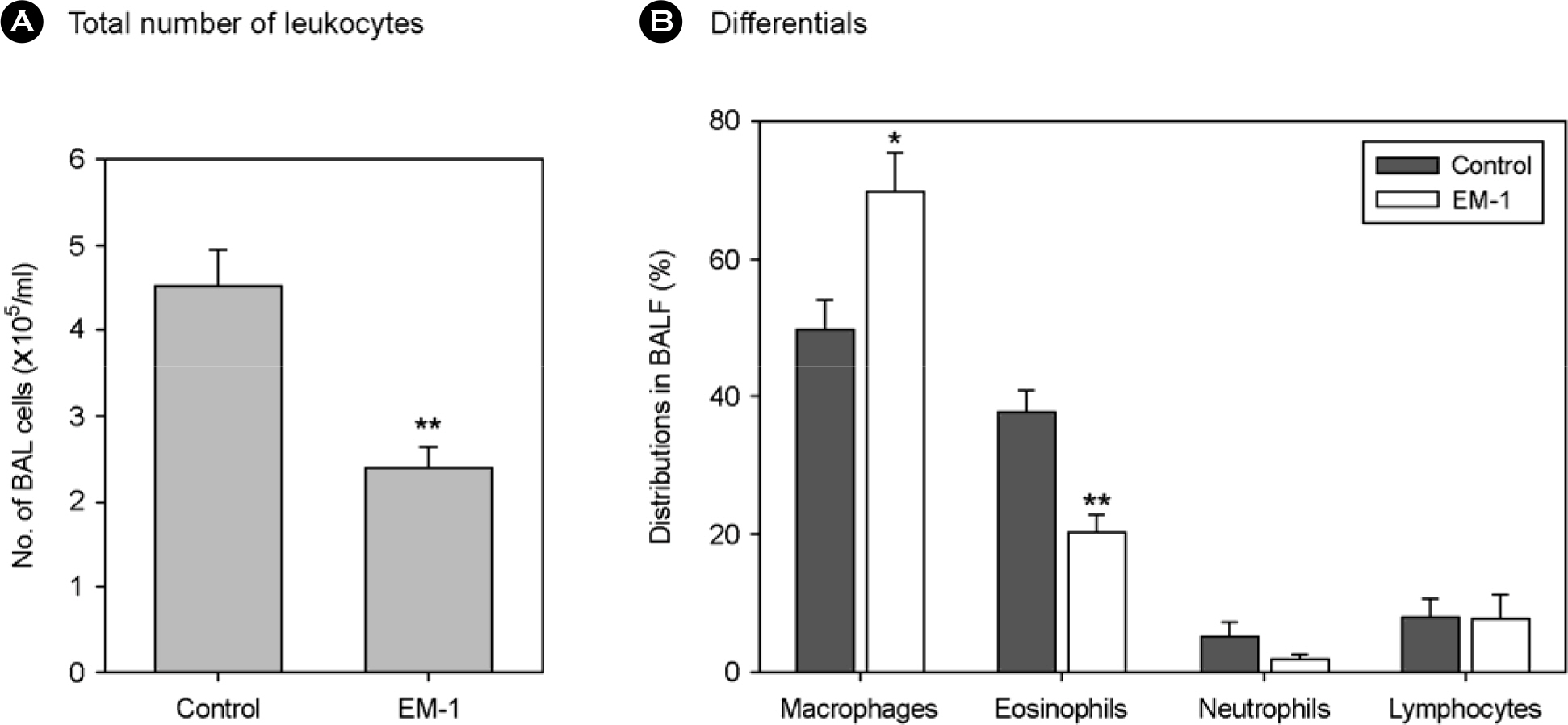
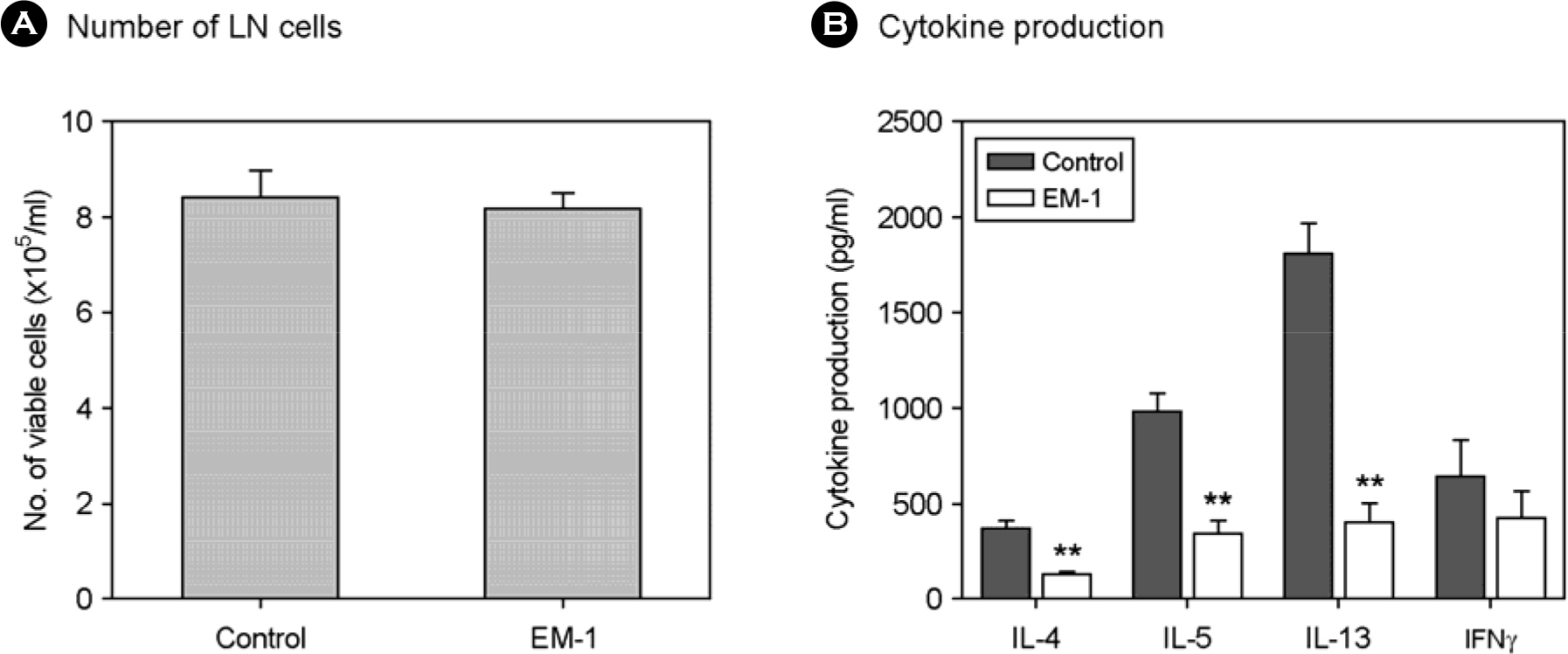
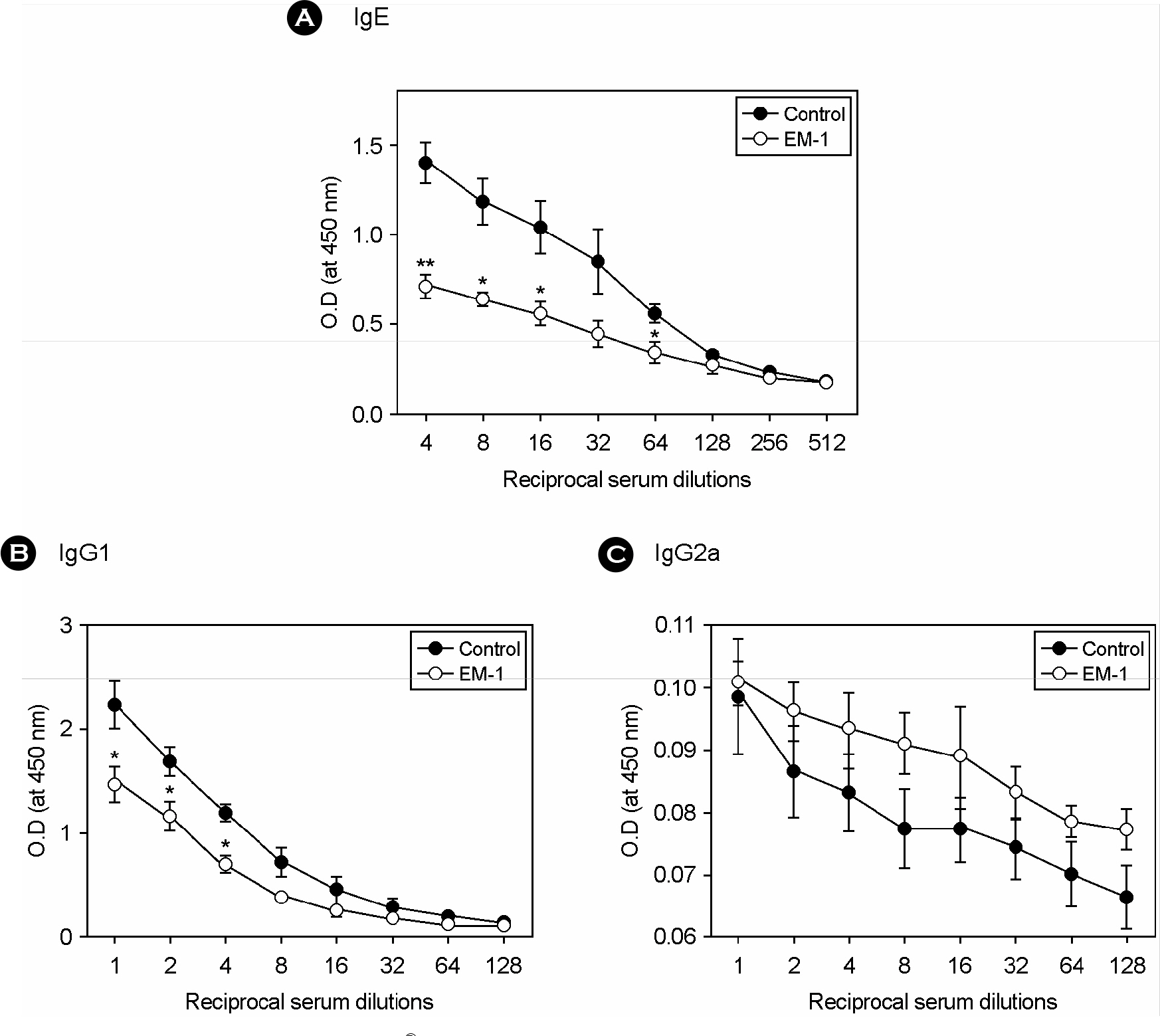
- Effective microorganism (EM) fermentation extract has been widely used for agricultural and environmental application. It has been recently revealed that EM cocktail treatment may be effective for treatment of diseases including cancer. In the present study, effectiveness of EM cocktail to control asthma was investigated using a mouse model of allergic asthma. Asthmatic mice sensitized and intranasally challenged with OVA were orally given EM fermentate (EM-1(R) during antigen challenge. Administration of EM-1(R) resulted in a significant reduction in airway hyper-reactivity (AHR) and airway recruitment of total leukocytes and eosinophils. Cytokine (IL-4, IL-5 and IFNgamma) levels in bronchoalveolar lavage fluid (BALF) and lung tissues were not altered by EM-1(R) treatment. However, IL-13 level in BALF was considerably lower in EM-1(R) treated mice than in controls. Moreover, Ag-specific IL-4, IL-5 and IL-13 production of draining lymph node cells were markedly downregulated by EM-1(R) treatment when compared to controls, whereas their IFNgamma production was not significantly different. Those data show that EM-1(R) treatment suppresses type 2 helper T (Th2), but not type 1 helper T (Th1), cell response. This finding was also supported by serum antibody data showing that IgE and IgG1 levels in EM-1(R) treated mice were significantly lower than in controls, while IgG2a level was not significantly different between two groups. In conclusion, oral administration of EM-1(R) attenuates asthmatic manifestations including AHR and airway recruitment of eosinophils in a mouse model and which possibly results from selective inhibition of Th2 cell response to allergen. Our data also suggest that EM-1(R) may be effectively applied for control of allergic asthma.
MeSH Terms
Figure
Reference
-
References
1). Adelroth E, Rosenhall L, Glennow C. High dose inhaled budesonide in the treatment of severe steroid-dependent asthmatics. A two-year study. Allergy. 40:58–64. 1985.2). Aruoma OI, Deiana M, Rosa A, Casu V, Piga R, Peccagnini S, Dessi MA, Ke B, Liang YF, Higa T. Assessment of the ability of the antioxidant cocktail-derived from fermentation of plants with effective microorganisms (EM-X) to modulate oxidative damage in the kidney and liver of rats in vivo: studies upon the profile of poly- and mono-unsaturated fatty acids. Toxicol Lett. 135:209–217. 2002.
Article3). Aruoma OI, Moncaster JA, Walsh DT, Gentleman SM, Ke B, Liang YF, Higa T, Jen LS. The antioxidant cocktail, effective microorganism X (EM-X), protects retinal neurons in rats against N-methyl-D-aspartate excitotoxicity in vivo. Free Radic Res. 37:91–97. 2003.4). Bryan SA, Leckie MJ, Hansel TT, Barnes PJ. Novel therapy for asthma. Expert Opin Investig Drugs. 9:25–42. 2000.5). Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 24:73–80. 1994.
Article6). Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 320:271–277. 1989.
Article7). Busse WW, Sedgwick JB. Eosinophils in asthma. Ann Allergy. 68:286–290. 1992.8). Busse WW, Lemanske RF. Asthma. N Eng J Med. 344:350–362. 2001.
Article9). Caramori G, Adcock I. Pharmacology of airway inflammation in asthma and COPD. Pulm Pharmacol Ther. 16:247–277. 2003.
Article10). Chui CH, Cheng GY, Ke B, Lau FY, Wong RS, Kok SH, Fatima S, Cheung F, Cheng CH, Chan AS, Tang JC. Growth inhibitory potential of effective microorganism fermentation extract (EM-X) on cancer cells. Int J Mol Med. 14:925–929. 2004.
Article11). Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 54:825–857. 1999.
Article12). Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 141:970–977. 1990.
Article13). Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 183:109–117. 1996.
Article14). Coyle AJ, Le Gros G, Bertrand C, Tsuyuki S, Heusser CH, Kopf M, Anderson GP. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 13:54–59. 1995.
Article15). Datla KP, Bennett RD, Zbarsky V, Ke B, Liang YF, Higa T, Bahorun T, Aruoma OI, Dexter DT. The antioxidant drink effective microorganism-X (EM-X) pre-treatment attenuates the loss of nigrostriatal dopaminergic neurons in 6-hydroxydopamine-lesion rat model of Parkinson's disease. J Pharm Pharmacol. 56:649–654. 2004.
Article16). Deiana M, Dessi MA, Ke B, Liang YF, Higa T, Gilmour PS, Jen LS, Rahman I, Aruoma OI. The antioxidant cocktail effective microorganism X (EM-X) inhibits oxidant-induced interleukin-8 release and the peroxidation of phospholipids in vitro. Biochem Biophys Res Commun. 296:1148–1151. 2002.
Article17). Del Prete G, Maggi E., Paola P., Ghretien I., Tiri A., Macchia D., Banchereau J., de Vries J. E., Romagnani S.IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatant. J Immunol. 140:4193–4198. 1988.18). Ferguson AC, Whitelaw M, Brown H. Correlation of bronchial eosinophil and mast cell activation with bronchial hyperresponsiveness in children with asthma. J Allergy Clin Immunol. 90:609–613. 1992.
Article19). Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 183:195–201. 1996.
Article20). Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, Nikander K, Persson T, Reinikainen K, Selroos O. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 325:388–392. 1991.21). Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CJ, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 156:766–775. 1997.
Article22). Hansel TT, Barnes PJ. Novel drugs for treating asthma. Curr Allergy Asthma Rep. 1:164–173. 2001.
Article23). Heebink L. Case reports on EM-X therapy on malignant disease. pp. 22–23. In. Abstracts for The Second International EM Medical Conference, International EM Medical Conference Executive Committee Secretariat. Okinawa, Japan,. 2003.24). Henderson WR Jr, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 100:3083–3092. 1997.
Article25). Higa T. Effective microorganisms: A biotechnology for mankind. pp. p. 8–14. In. Proceedings of the First International Conference on Kyusei Nature Farming. Parr JF, Hornick SB, Whitman CE, editors. (Ed.),. U.S. Department of Agriculture;Washington, D.C.: 1991.26). Higa T. Use of microorganisms in agriculture & their positive effects on environmental safety. Nobunkyo, Tokyo, Japan. 1995.27). Higa T. An earth saving revolution. Sunmark Publishing, Inc., Tokyo, Japan. 1996.28). Higa T. Latest development of EM technology. pp. 7–9. In. Abstracts for The Second International EM Medical Conference, International EM Medical Conference Executive Committee Secretariat. Okinawa, Japan,. 2003.29). Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 22:179–189. 2002.
Article30). Kay AB, Ying S, Varney V, Gaga M, Durham SR, Moqbel R, Wardlaw AJ, Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 173:775–778. 1991.
Article31). Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low-income urban children: preliminary findings from the Seattle-King County healthy homes project. J Urban Health. 77:50–67. 2000.
Article32). Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 46:111–147. 1989.
Article33). Nimmagadda SR, Spahn JD, Nelson HS, Jenkins J, Szefler SJ, Leung DY. Fluticasone propionate results in improved glucocorticoid receptor binding affinity and reduced oral glucocorticoid requirements in severe asthma. Ann Allergy Asthma Immunol. 81:35–40. 1998.
Article34). Oh J-S, Lee J-S, Kang K-H, Kim H-T, Chung W-B, Jeong S-J. Effect of Microbial Fermentation Compost by Cultivating Area. Kor J Org Agricul. 10:87–97. 2002.35). Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 77:1859–1870. 1991.
Article36). Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 326:298–304. 1992.
Article37). Rothenberg ME. Eosinophilia. N Engl J Med. 338:1592–1600. 1998.
Article38). Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA Jr. Corticosteroid therapy for acute asthma. Respir Med. 98:275–284. 2004.
Article39). Salmeron S, Guerin JC, Godard P, Renon D, Henry-Amar M, Duroux P, Taytard A. High doses of inhaled corticosteroids in unstable chronic asthma. A multicenter, double-blind, placebo-controlled study. Am Rev Respir Dis. 140:167–171. 1989.
Article40). Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 325:1067–1071. 1991.
Article41). Seminario MC, Gleich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 6:860–864. 1994.
Article42). Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O'Neill KR, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 170:3296–3305. 2003.
Article43). Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 275:77–79. 1997.
Article44). Simon HU, Blaser K. Inhibition of programmed eosinophil death: a key pathogenic event for eosinophilia? Immunol Today. 16:53–55. 1995.
Article45). Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 145:3796–3806. 1990.46). Walker C, Virchow JC Jr, Bruijnzeel PL, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 146:1829–1835. 1991.47). Warringa RAJ, Schwiezer RC, Maikoe T, Bruijnzeel PLB, Koenderman L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 7:631–636. 1992.
Article48). Weiss KB, Gergen PJ, Hodgson TA. An economic evaluation of asthma in the United States. N Engl J Med. 326:862–866. 1992.
Article49). Wills-Karp M. Murine models of asthma in understanding immune dysregulation in human asthma. Immunopharmacology. 48:263–268. 2000.
Article50). Yoshino Y, Takeda M, Sano K. Clinical efficacy of intravenous infusion of EM-X in 42 cases of terminal cancers. pp. p. 20–21. In. Abstracts for The Second International EM Medical Conference, International EM Medical Conference Executive Committee Secretariat. Okinawa, Japan,. 2003.51). Yuan HC. EM-X as a free radical scavenger. Center for Condensed Matter Science, National Taiwan University, Taipei. 1999.52). Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 15:19–26. 1994.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding asthma using animal models
- An Intratracheal Challenge Murine Model of Asthma: Can Bronchial Inflammation Affect the Nose?
- Anti-inflammatory effects of Rhizopus spp. fermentation products on ovalbumin-induced asthma in mice
- Ampicillin treated German cockroach extract leads to reduced inflammation in human lung cells and a mouse model of Asthma
- Effect of Route of Airway Allergen Challenge on Airway Inflammation and Hyperresponsiveness in Mouse Asthma Model