Allergy Asthma Immunol Res.
2015 Jan;7(1):76-82. 10.4168/aair.2015.7.1.76.
An Intratracheal Challenge Murine Model of Asthma: Can Bronchial Inflammation Affect the Nose?
- Affiliations
-
- 1State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. klai@163.com
- 2Department of Pathology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- KMID: 2260155
- DOI: http://doi.org/10.4168/aair.2015.7.1.76
Abstract
- PURPOSE
Extensive data support the influence of the upper airway on lower airway inflammation and pathophysiology in allergic disease. However, few studies have focused on allergic inflammation in the nose after an isolated lower airway allergen challenge, a situation that can exist clinically when human subjects breathe primarily through the mouth, as occurs when nasally congested. This study used a mouse model to investigate whether upper airway inflammation and hyperresponsiveness were induced by an isolated lower airway allergen challenge.
METHODS
BALB/c mice were sensitized by systemic intraperitoneal injection of ovalbumin/saline and challenged with intratracheal ovalbumin/saline. Inflammation in the nose and lungs was assessed by cytology and histology of nasal tissues and bronchoalveolar lavage fluid (BALF), while nasal airway resistance and response were measured over 3 days post-challenge.
RESULTS
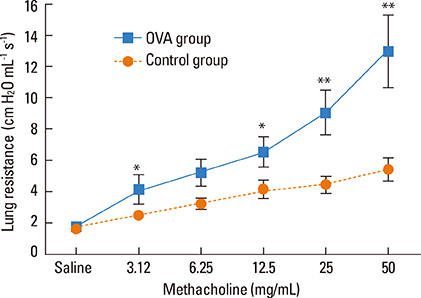
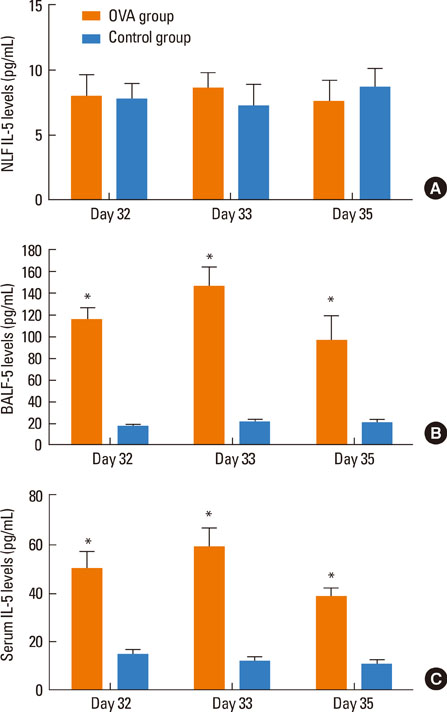
Intratracheal application of an allergen in anaesthetized mice resulted in exclusive deposition in the lower airway. Compared to control animals, ovalbumin-sensitized mice after challenge showed bronchial hyperreactivity and increased IL-5 in the serum BALF, as well as eosinophil infiltration in the lungs. However, nasal histology of the ovalbumin-sensitized mice showed no increase in eosinophil infiltration. The nasal lavage fluid revealed no increase in eosinophils or IL-5, and the nasal airway resistance did not increase after challenge either.
CONCLUSIONS
In a mouse allergy model, exclusive allergen challenge of the lower airway can elicit a pulmonary and systemic allergic response, but does not induce upper airway inflammatory or physiological responses.
Keyword
MeSH Terms
Figure
Reference
-
1. Pawankar R, Takizawa R. Revisiting the link between allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2007; 7:77–78.2. Fasano MB. Combined airways: impact of upper airway on lower airway. Curr Opin Otolaryngol Head Neck Surg. 2010; 18:15–20.3. Krouse JH, Brown RW, Fineman SM, Han JK, Heller AJ, Joe S, et al. Asthma and the unified airway. Otolaryngol Head Neck Surg. 2007; 136:S75–S106.4. Leynaert B, Neukirch C, Kony S, Guénégou A, Bousquet J, Aubier M, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004; 113:86–93.5. Brown JL, Behndig AF, Sekerel BE, Pourazar J, Blomberg A, Kelly FJ, et al. Lower airways inflammation in allergic rhinitics: a comparison with asthmatics and normal controls. Clin Exp Allergy. 2007; 37:688–695.6. Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994; 15:21–25.7. Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001; 107:469–476.8. Bonay M, Neukirch C, Grandsaigne M, Leçon-Malas V, Ravaud P, Dehoux M, et al. Changes in airway inflammation following nasal allergic challenge in patients with seasonal rhinitis. Allergy. 2006; 61:111–118.9. Halpern MT, Schmier JK, Richner R, Guo C, Togias A. Allergic rhinitis: a potential cause of increased asthma medication use, costs, and morbidity. J Asthma. 2004; 41:117–126.10. Corren J. The connection between allergic rhinitis and bronchial asthma. Curr Opin Pulm Med. 2007; 13:13–18.11. McCusker C, Chicoine M, Hamid Q, Mazer B. Site-specific sensitization in a murine model of allergic rhinitis: role of the upper airway in lower airways disease. J Allergy Clin Immunol. 2002; 110:891–898.12. Wang Y, McCusker CT. Interleukin-13-dependent bronchial hyper-responsiveness following isolated upper-airway allergen challenge in a murine model of allergic rhinitis and asthma. Clin Exp Allergy. 2005; 35:1104–1111.13. KleinJan A, Willart M, van Nimwegen M, Leman K, Hoogsteden HC, Hendriks RW, et al. United airways: circulating Th2 effector cells in an allergic rhinitis model are responsible for promoting lower airways inflammation. Clin Exp Allergy. 2010; 40:494–504.14. Hens G, Raap U, Vanoirbeek J, Meyts I, Callebaut I, Verbinnen B, et al. Selective nasal allergen provocation induces substance P-mediated bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2011; 44:517–523.15. Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000; 161:2051–2057.16. Li J, Saito H, Crawford L, Inman MD, Cyr MM, Denburg JA. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005; 114:386–396.17. Hellings PW, Hessel EM, Van Den Oord JJ, Kasran A, Van Hecke P, Ceuppens JL. Eosinophilic rhinitis accompanies the development of lower airway inflammation and hyper-reactivity in sensitized mice exposed to aerosolized allergen. Clin Exp Allergy. 2001; 31:782–790.18. Zhang Q, Lai K, Xie J, Chen G, Zhong N. Does unrestrained single-chamber plethysmography provide a valid assessment of airway responsiveness in allergic BALB/c mice? Respir Res. 2009; 10:61.19. Xie J, Zhang Q, Lai K, Zhong N. Measurement of nasal airway resistance and response in mice. Int Arch Allergy Immunol. 2010; 151:262–264.20. Tsunematsu M, Yamaji T, Kozutsumi D, Murakami R, Kimura S, Kino K. Establishment of an allergic rhinitis model in mice for the evaluation of nasal symptoms. Life Sci. 2007; 80:1388–1394.21. Hens G, Bobic S, Reekmans K, Ceuppens JL, Hellings PW. Rapid systemic uptake of allergens through the respiratory mucosa. J Allergy Clin Immunol. 2007; 120:472–474.22. Braunstahl GJ, Hellings PW. Allergic rhinitis and asthma: the link further unraveled. Curr Opin Pulm Med. 2003; 9:46–51.23. Gherson G, Moscato G, Vidi I, Salvaterra A, Candura F. Non-specific nasal reactivity: a proposed method of study. Eur J Respir Dis. 1986; 69:24–28.24. Demoly P, Bousquet PJ. Links between allergic rhinitis and asthma still reinforced. Allergy. 2008; 63:251–254.25. Hens G, Hellings PW. The nose: gatekeeper and trigger of bronchial disease. Rhinology. 2006; 44:179–187.26. Rimmer J, Ruhno JW. 6: Rhinitis and asthma: united airway disease. Med J Aust. 2006; 185:565–571.27. McCusker CT. Use of mouse models of allergic rhinitis to study the upper and lower airway link. Curr Opin Allergy Clin Immunol. 2004; 4:11–16.28. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003; 111:1171–1183.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effectiveness of Pimecrolimus in Airway Inflammation and Bronchial Hyperresponsiveness in Murine Asthma Model
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Effect of Route of Airway Allergen Challenge on Airway Inflammation and Hyperresponsiveness in Mouse Asthma Model
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma
- Changes of soluble ICAM- 1 levels in serum and bronchoalveolar lavage fluid from patients with atopic bronchial asthma after allergen challenge