J Korean Med Sci.
2005 Aug;20(4):535-541. 10.3346/jkms.2005.20.4.535.
Differential Effect of gamma-radiation-induced Heme Oxygenase-1 Activity in Female and Male C57BL/6 Mice
- Affiliations
-
- 1Laboratory of Radiation Immunology, Korea Institute of Radiological & Medical Sciences, KAERI, Seoul, Korea. immu@kcch.re.kr
- 2Laboratory of Radiation Biophysics, Department of Biophysics, Biological Faculty, Moscow State University, Moscow, Russia.
- KMID: 1712729
- DOI: http://doi.org/10.3346/jkms.2005.20.4.535
Abstract
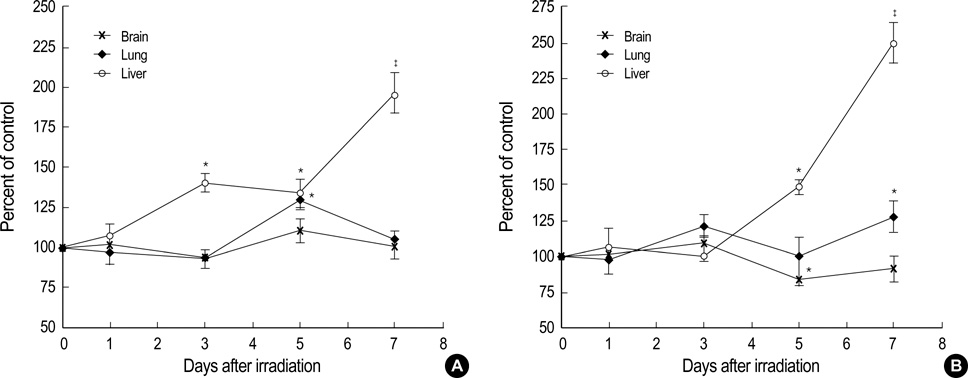
- Ionizing radiation produces reactive oxygen species, which exert diverse biological effects on cells and animals. We investigated alterations of heme oxygenase (HO) and non-protein thiols (NPSH), which are known as two major anti-oxidant enzymes, in female and male C57BL/6 mice in the lung, liver, and brain after whole-body gamma-irradiation with 10 Gy (1-7 days) as well as in the lung after whole-thorax gamma-irradiation (WTI) with 12.5 Gy (1-26 weeks). Most significant alteration of HO activity was observed in the liver, which elevated 250% in males. NPSH level in female liver was increased on the 5th-7th days but decreased in males on the 3rd day. In the lung, the elevation of HO activity in both sexes and the pattern of NPSH change were similar to that of the liver. On the other hand, the increase of HO activity on the 16th week and the decrease of NPSH level on the 2nd week were observed only in male lung after WTI. This study shows that the liver is the most sensitive tissue to gamma-irradiation-induced alterations of HO activity in both female and male mice. In addition, there exists significant differential effect of gamma-irradiation on anti-oxidant system in female and male mice.
Keyword
MeSH Terms
-
Animals
Brain/*enzymology/metabolism/radiation effects
Comparative Study
Female
Gamma Rays
Gene Expression Regulation, Enzymologic/radiation effects
Liver/*enzymology/metabolism/radiation effects
Lung/*enzymology/metabolism/radiation effects
Male
Mice
Mice, Inbred C57BL
RNA, Messenger/genetics/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Sex Factors
Sulfhydryl Compounds/metabolism
Time Factors
Whole-Body Irradiation
Figure
Reference
-
1. Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000. 28:289–309.2. Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1029–L1037.
Article3. Taketani S, Kohno H, Yoshinaga T, Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989. 245:173–176.
Article4. Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989. 86:99–103.
Article5. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988. 2:2557–2568.
Article6. Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996. 6:129–168.
Article7. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987. 235:1043–1046.
Article8. Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000. 348:615–619.
Article9. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.10. Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994. 91:2607–2610.
Article11. Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000. 28:1405–1420.
Article12. Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002. 64:1019–1026.
Article13. Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001. 8:763–772.
Article14. Sedlak J, Hanus L. Changes of glutathione and protein bound SH-groups concentration in rat adrenals under acute and repeated stress. Endocrinol Exp. 1982. 16:103–109.15. Sedlak J. Long-term effect of hypophysectomy on various fractions of sulfhydryl groups in thyroid, adrenal and some other organs in rats. Endocrinol Exp. 1985. 19:186–192.16. Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000. 10:296–307.
Article17. Schacterle GR, Pollack RL. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973. 51:654–655.
Article18. Maines MD, Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978. 253:2321–2326.
Article19. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968. 25:192–205.
Article20. Deev LI, Topchishvili GI, Akhalaia MI, Platonov AG. Effect of X-ray irradiation on the activity of key enzymes in heme biosynthesis and breakdown in the rat liver. Biull Eksp Biol Med. 1985. 99:681–683.21. Suzuki K, Mori M, Kugawa F, Ishihara H. Whole-body X-irradiation induces acute and transient expression of heme oxygenase-1 in rat liver. J Radiat Res (Tokyo). 2002. 43:205–210.
Article22. Datta PK, Moulder JE, Fish BL, Cohen EP, Lianos EA. Induction of heme oxygenase 1 in radiation nephropathy: role of angiotensin II. Radiat Res. 2001. 155:734–739.
Article23. Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991. 51:974–978.24. Dennery PA, Rodgers PA, Lum MA, Jennings BC, Shokoohi V. Hyperoxic regulation of lung heme oxygenase in neonatal rats. Pediatr Res. 1996. 40:815–821.
Article25. Johnston CJ, Wright TW, Rubin P, Finkelstein JN. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and -sensitive mice after thoracic irradiation. Exp Lung Res. 1998. 24:321–337.
Article25. Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992. 13:227–232.
Article26. Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000. 47:1033–1042.26. Willis D, Moore AR, Willoughby DA. Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol. 2000. 190:627–634.
Article27. Rizzardini M, Terao M, Falciani F, Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993. 290:343–347.
Article28. Morse D. The role of heme oxygenase-1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003. 29:Supple 3. S82–S86.29. Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000. 48:89–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Change of Expression and Activity of Heme Oxygenase-1 in Rat Corpus Cavernosum during Low-flow Priapism
- Caffeic acid phenethyl ester protects against photothrombotic cortical ischemic injury in mice
- Iron Contents in Rice Food Derived from the Iron Pot, and In Vitro Study Regarding Heme Oxygenase-1 Activity
- Soluble factor from tumor cells induces heme oxygenase-1 by a nitric oxide-independent mechanism in murine peritoneal macrophages