J Korean Soc Endocrinol.
2006 Apr;21(2):125-131. 10.3803/jkes.2008.21.2.125.
Role of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the Growth Regulation of Anaplastic Thyroid Cancer Cells Lines
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Korea.
- 2Asan Institute for Life Sciences, University of Ulsan, Korea.
- 3Department of Internal Medicine, Maryknoll Hospital, Korea.
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Korea.
- KMID: 1511951
- DOI: http://doi.org/10.3803/jkes.2008.21.2.125
Abstract
-
BACKGROUND: Anaplastic thyroid carcinoma is one of the most aggressive human cancers with a median survival of only 6 months. Local surgical tumor debulking combined with radio-chemotherapy is generally used to treat this malady, but the low success rate has prompted the search for new therapeutic targets. We used 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) as an AMP-activated protein kinase (AMPK) activator to induce growth suppression and apoptosis in the anaplastic thyroid carcinoma cells.
METHODS
We investigated the effect of AICAR on the proliferation of thyroid cancer cell lines (ARO, WRO and FRO) by performing methyl-thiazoletetrazolium bromide assay. We wanted to see the effect of AICAR on the apoptosis and cell cycle of the thyroid cancer cells, and we wanted to determine the mechanism of these changes.
RESULTS
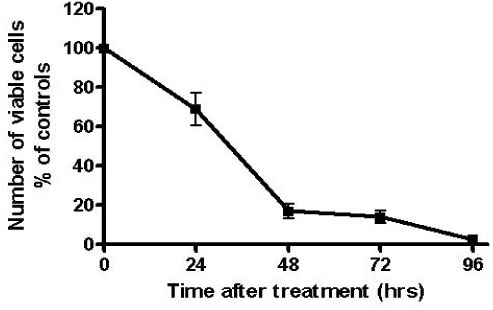
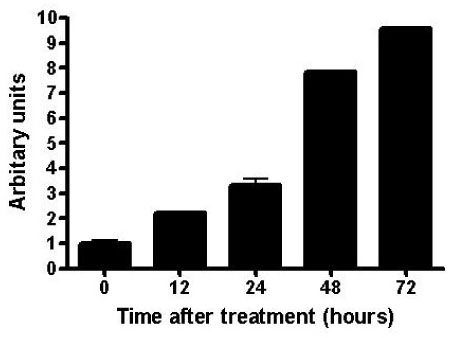
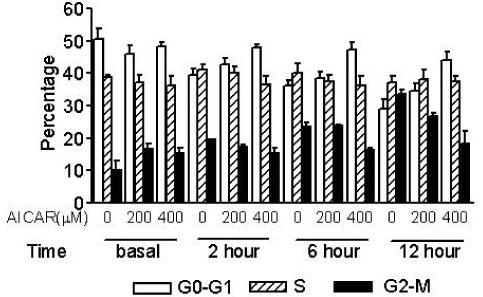
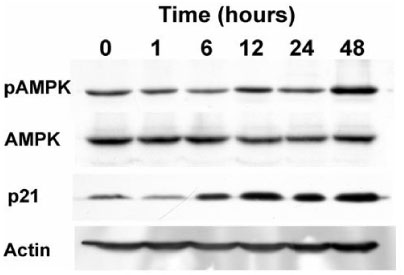
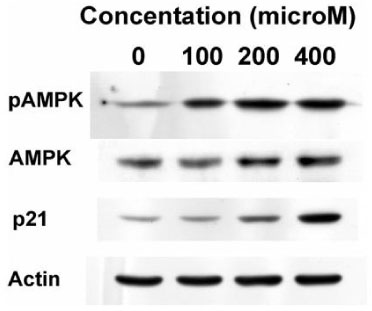
The proliferation of all thyroid cancer cell lines was significantly inhibited by administration of AICAR. FRO was the most susceptible cell line to AICAR treatment and so further studies were then performed with this cell line. The suppressive effect of AICAR on cell proliferation was related with phosphorylation of AMPK and the increased apoptosis. Also, cell cycle analysis revealed that progression to the G2-M phase was arrested (S-phase arrest) by AICAR treatment. S-phase arrest was associated with the increased protein expression of p21.
CONCLUSION
In the anaplastic thyroid cancer cell lines, AICAR inhibited proliferation due to the arrest in the S-phase; this was accompanied with the increased expression of p21. Overall, AMPK activation by AICAR or any other pharmacological agent could be a tempting potential target for thyroid cancer therapy.
MeSH Terms
Figure
Reference
-
2. Pasieka JL. Anaplastic thyroid cancer. Curr Opin Oncol. 2003. 15:78–83.3. Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001. 27:260–264.4. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001. 130:1028–1034.5. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg. 2001. 25:617–622.6. Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003. 546:113–120.7. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998. 67:821–855.8. Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997. 246:259–273.9. Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002. 21:6082–6090.10. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995. 229:558–565.11. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003. 115:577–590.12. Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-{beta}-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005. 280:39582–39593.13. Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004. 321:161–167.14. Russell RR III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004. 114:495–503.15. Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004. 61:610–619.16. Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001. 489:149–153.17. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002. 51:159–167.18. Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004. 67:2005–2011.19. Li J, Jiang P, Robinson M, Lawrence TS, Sun Y. AMPK-beta1 subunit is a p53-independent stress responsive protein that inhibits tumor cell growth upon forced expression. Carcinogenesis. 2003. 24:827–834.20. Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, Van de CM. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003. 30:151–161.21. Meisse D, Van de CM, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002. 526:38–42.22. Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, Tozzi MG. 5'-aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience. 2003. 117:811–820.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Berberine Inhibited the Growth of Thyroid Cancer Cell Lines 8505C and TPC1
- The effect of 5-aminoimidazole-4-carboxamide-ribonucleoside was mediated by p38 mitogen activated protein kinase signaling pathway in FRO thyroid cancer cells
- The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma
- Treatment Effect of Combining Lenvatinib and Vemurafenib for BRAF Mutated Anaplastic Thyroid Cancer
- An Unusual Case of Metastatic Non-Small Cell Lung Cancer Misidentified as Anaplastic Thyroid Cancer