J Bacteriol Virol.
2009 Sep;39(3):205-216. 10.4167/jbv.2009.39.3.205.
Expression of Endothelin-1 by Stimulation with CXCL8 in Mouse Peritoneal Macrophages
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Yeungnam University, Daegu, Korea.
- 2Department of Microbiology, College of Medicine, Yeungnam University, Daegu, Korea. heesun@med.yu.ac.kr
- 3Department of Emergency Medicine, College of Medicine, Inha University, Incheon, Korea.
- KMID: 1474163
- DOI: http://doi.org/10.4167/jbv.2009.39.3.205
Abstract
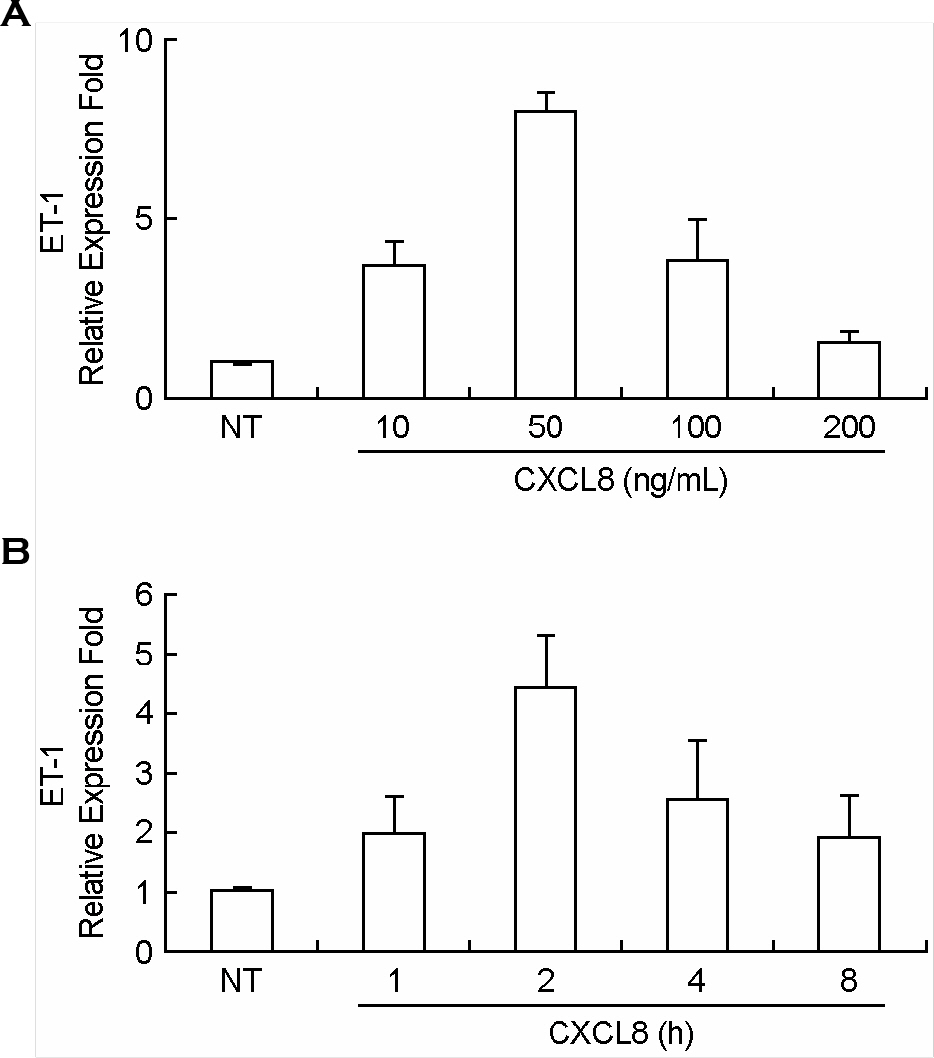
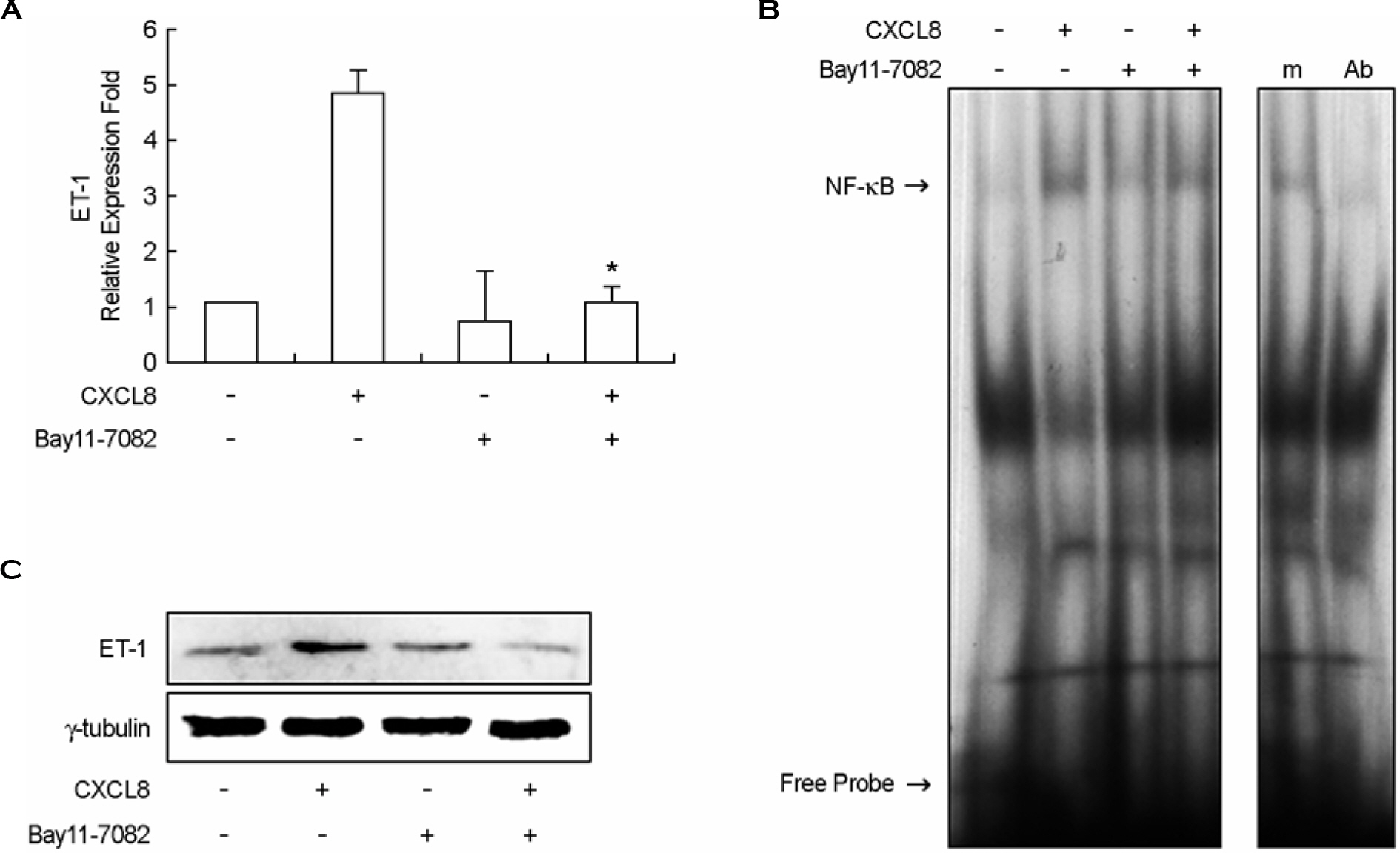
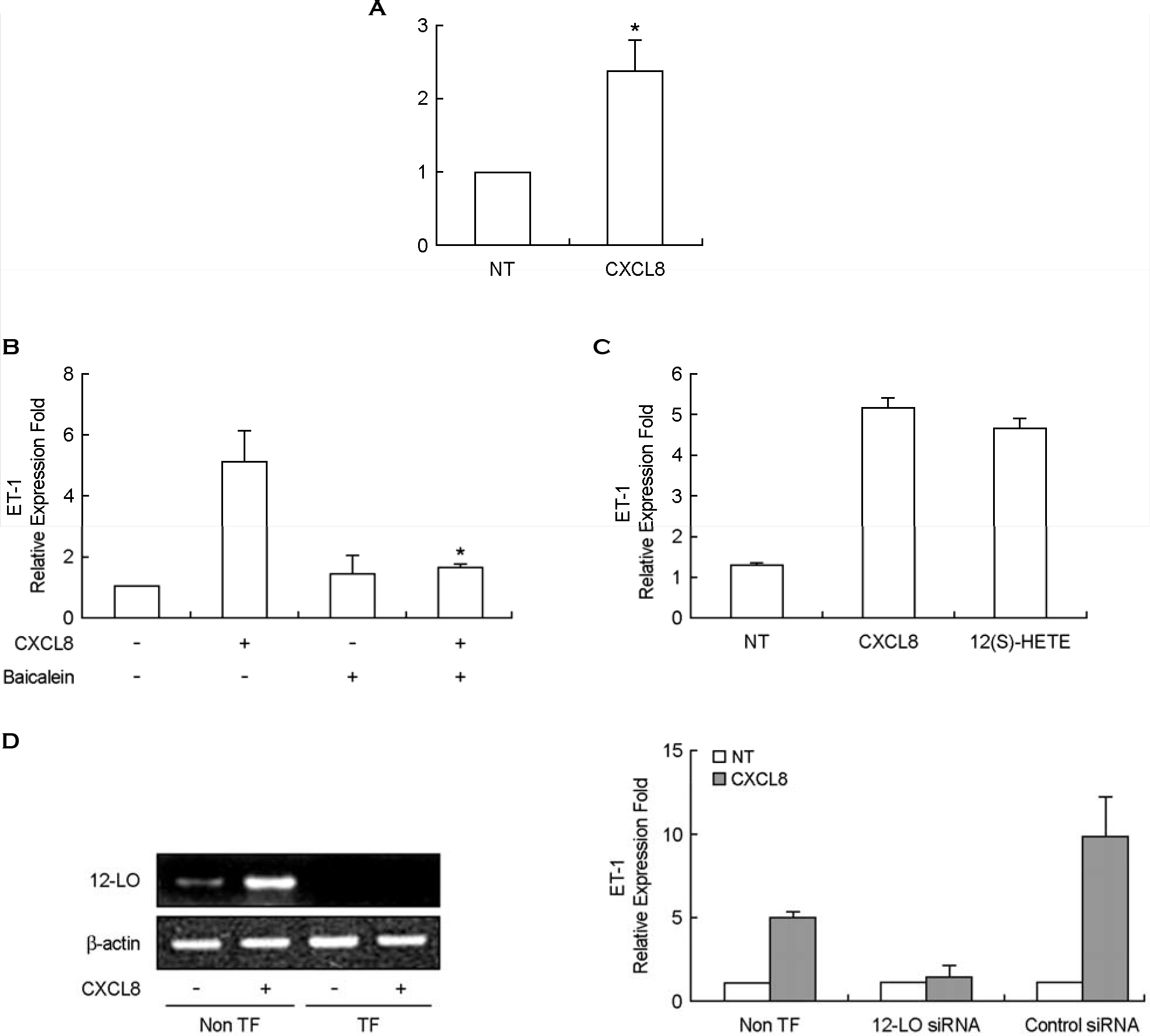
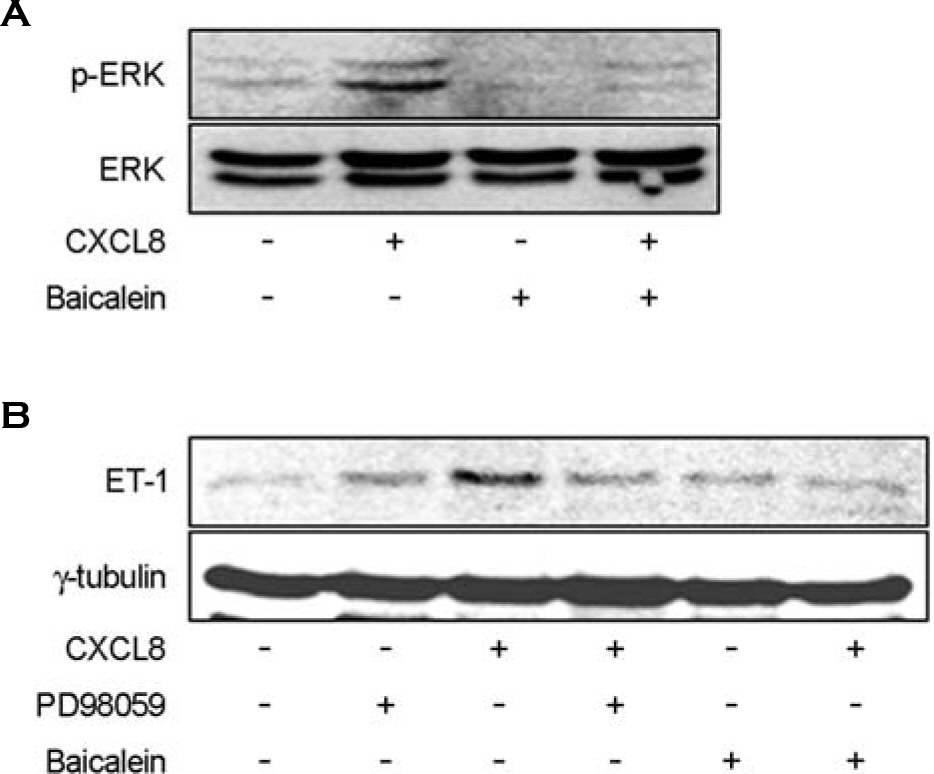
- Endothelin-1 (ET-1) has been characterized as a potent vasoconstrictor secreted by the endothelium, and play a major role in the regulation of vascular tone. It has been also known to participate in inflammatory reactions. The production of ET-1 by macrophages during infection and inflammation is related to tissue perfusion and leukocyte extravasation. The aim of this study is to investigate the role of IL-8/CXCL8, as a major inflammatory chemokine, for ET-1 expression in macrophges. Expression of ET-1 mRNA in mouse peritoneal macrophages (PeM phi) was weaker than that in vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats (WKY). However, expression of IL-8/CXCL8-induced ET-1 mRNA in PeM phi was much more stronger than that in SHR and WKY VSMCs. Maximum expression of ET-1 mRNA was observed at 50 ng/ml dose of IL-8/CXCL8 and occurred at 2 h after addition of IL-8/CXCL8. Expression of ET-1 by IL-8/CXCL8 was dependent on NF-kappaB activation and ERK1/2 phosphorylation. Baicalein, a 12-lipoxygenase (LO) inhibitor, inhibited the expression of IL-8/CXCL8-induced ET-1 mRNA. This inhibitory action of baicalein was mediated via ERK1/2 inactivation. Induction of 12-LO mRNA by IL-8/CXCL8 and expression of ET-1 mRNA by 12-LO metabolite, 12(S)-HETE were also detected. The expression of IL-8/CXCL8-induced ET-1 mRNA was not detected in PeM phi transfected with 12-LO siRNA. These results suggest that IL-8/CXCL8 can act as one of main inducers of ET-1 in vascular inflammatory reactions, and ET-1 expression by IL-8/CXCL8 is related to 12-LO pathway in PeM phi.
MeSH Terms
-
Animals
Arachidonate 12-Lipoxygenase
Endothelin-1
Endothelium
Flavanones
Inflammation
Leukocytes
Macrophages
Macrophages, Peritoneal
Mice
Muscle, Smooth, Vascular
NF-kappa B
Perfusion
Phosphorylation
Rats
Rats, Inbred SHR
RNA, Messenger
RNA, Small Interfering
Arachidonate 12-Lipoxygenase
Endothelin-1
Flavanones
NF-kappa B
RNA, Messenger
RNA, Small Interfering
Figure
Cited by 1 articles
-
CCL5 Inhibits Elevation of Blood Pressure and Expression of Hypertensive Mediators in Developing Hypertension State Spontaneously Hypertensive Rats
Hye Young Kim, Hye Ju Cha, Jin Hee Choi, Young Jin Kang, So Young Park, Hee Sun Kim
J Bacteriol Virol. 2015;45(2):138-150. doi: 10.4167/jbv.2015.45.2.138.
Reference
-
1). Agapitov AV., Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2002. 3:1–15.
Article2). Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–5.
Article3). Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989. 86:2863–7.
Article4). Inoue A., Yanagisawa M., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem. 1989. 264:14954–9.5). Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005. 43:19–29.
Article6). Battistini B., Forget MA., Laight D. Potential roles for endothelins in systemic inflammatory response syndrome with a particular relationship to cytokines. Shock. 1996. 5:167–83.
Article7). Wanecek M., Weitzberg E., Rudehill A., Oldner A. The endothelin system in septic and endotoxin shock. Eur J Pharmacol. 2000. 407:1–15.
Article8). Kedzierski RM., Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001. 41:851–76.
Article9). Bousette N., Giaid A. Endothelin-1 in atherosclerosis and other vasculopathies. Can J Physiol Pharmacol. 2003. 81:578–87.
Article10). Black SM., Kumar S., Wiseman D., Ravi K., Wedgwood S., Ryzhov V., Fineman JR. Pediatric pulmonary hypertension: Roles of endothelin-1 and nitric oxide. Clin Hemorheol Microcirc. 2007. 37:111–20.11). Ebihara I., Nakamura T., Shimada N., Shoji H., Koide H. Effect of hemoperfusion with polymyxin B-immobilized fiber on plasma endothelin-1 and endothelin-1 mRNA in monocytes from patients with sepsis. Am J Kidney Dis. 1998. 32:953–61.
Article12). Liu B., Zhou J., Chen H., Wang D., Hu D., Wen Y., Xiao N. Expression and cellular location of endothelin-1 mRNA in rat liver following endotoxemia. Chin Med J. 1997. 110:932–5.13). Lundblad R., Giercksky KE. Endothelin concentrations in experimental sepsis: profiles of big endothelin and endothelin 1-21 in lethal peritonitis in rats. Eur J Surg. 1995. 161:9–16.14). Wahl JR., Goetsch NJ., Young HJ., Van Maanen RJ., Johnson JD., Pea AS., Brittingham A. Murine macrophages produce endothelin-1 after microbial stimulation. Exp Biol Med. 2005. 230:652–8.
Article15). Thomson AW., Lotze MT. The Cytokine Handbook. Academic Press. 4th ed.San Diego: Elsevier Science;2003. p.10-1, p.p. 1056–7.16). Boekholdt SM., Peters RJ., Hack CE., Day NE., Luben R., Bingham SA., Wareham NJ., Reitsma PH., Khaw KT. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004. 24:1503–8.17). Kim HY., Kang YJ., Song IH., Choi HC., Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008. 31:515–23.
Article18). Ehrenreich H., Anderson RW., Fox CH., Rieckmann P., Hoffman GS., Travis WD., Coligan JE., Kehrl JH., Fauci AS. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med. 1990. 172:1741–8.
Article19). Ehrenreich H., Burd PR., Rottem M., Hultner L., Hylton JB., Garfield M., Coligan JE., Metcalfe DD., Fauci AS. Endothelins belong to the assortment of mast cell-derived and mast cell-bound cytokines. New Biol. 1992. 4:147–56.20). Ehrenreich H., Rieckmann P., Sinowatz F., Weih KA., Arthur LO., Goebel FD., Burd PR., Coligan JE., Clouse KA. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol. 1993. 150:4601–9.21). Xu J., Zhong NS. The interaction of tumour necrosis factor alpha and endothelin-1 in pathogenetic models of asthma. Clin Exp Allergy. 1997. 27:568–73.
Article22). Salh B., Hoeflick K., Kwan W., Pelech S. Granulocyte-macrophage colony-stimulating factor and interleukin-3 potentiate interferon-gamma-mediated endothelin production by human monocytes: role of protein kinase C. Immunology. 1998. 95:473–9.23). Kim HY., Kim HK., Kim JR., Kim HS. Upregulation of LPS-induced chemokine KC expression by 15-deoxy-delta12,14-prostaglandin J2 in mouse peritoneal macrophages. Immunol Cell Biol. 2005. 83:286–93.24). Griendling KK., Taubman MB., Akers M., Mendlowitz M., Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991. 266:15498–504.
Article25). Anggrahini DW., Emoto N., Nakayama K., Widyantoro B., Adiarto S., Iwasa N., Nonaka H., Rikitake Y., Kisanuki YY., Yanagisawa M., Hirata K. Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovasc Res. 2009. 82:143–51.
Article26). Tsai CS., Loh SH., Liu JC., Lin JW., Chen YL., Chen CH., Cheng TH. Urotensin II-induced endothelin-1 expression and cell proliferation via epidermal growth factor receptor trans-activation in rat aortic smooth muscle cells. Proceedings of Atherosclerosis. 2009.
Article27). Badr KF., Murray JJ., Breyer MD., Takahashi K., Inagami T., Harris RC. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney: elucidation of signal transduction pathways. J Clin Invest. 1989. 83:336–42.
Article28). Ito H., Hirata Y., Hiroe M., Tsujino M., Adachi S., Takamoto T., Nitta M., Taniguchi K., Marumo F. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Circ Res. 1991. 69:209–15.
Article29). Cunningham ME., Huribal M., Bala RJ., McMillen MA. Endothelin-1 and endothelin-4 stimulate monocyte production of cytokines. Crit Care Med. 1997. 25:958–64.
Article30). Liu G., Wang H., Ou D., Huang H., Liao D. Endothelin-1, an important mitogen of smooth muscle cells of spontaneously hypertensive rats. Chin Med J. 2002. 115:750–2.31). Hong HJ., Chan P., Liu JC., Juan SH., Huang MT., Lin JG., Cheng TH. Angiotensin II induces endothelin-1 gene expression via extracellular signal-regulated kinase pathway in rat aortic smooth msucle cells. Cardiovasc Res. 2004. 61:159–68.32). Chao HH., Liu JC., Lin JW., Chen CH., Wu CH., Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008. 29:1301–12.
Article33). Molet S., Furukawa K., Maghazechi A., Hamid Q., Giaid A. Chemokine- and cytokine-induced expression of endothelin 1 and endothelin-converting enzyme 1 in endothelial cells. J Allergy Clin Immunol. 2000. 105:333–8.
Article34). Cheng M., Li Y., Wu J., Nie Y., Li L., Liu X., Charoude HN., Chen H. IL-8 induces imbalances between nitric oxide and endothein-1 and also between plasminogen activator inhibitor-1 and tissue-type plasminogen activator in cultured endothelial cells. Cytokine. 2008. 41:9–15.35). Ros J., Leivas A., Jimenez W., Morales M., Bosch-Marce M., Arroyo V., Rivera F., Rodes J. Effect of bacterial lipopoly-saccharide on endothelin-1 production in human vascular endothelial cells. J Hepatol. 1997. 26:81–7.
Article36). Deshpande R., Khalili H., Pergolizzi RG., Michael SD., Chang MD. Estradiol down-regulates LPS-induced cytokine production and NF-κB activation in murine macrophages. Am J Reprod Immunol. 1997. 38:46–54.37). Kim HY., Kim JR., Kim HS. Upregulation of lipopolysaccharide-induced interleukin-10 by prostaglandin A1 in mouse peritoneal macrophages. J Microbiol Biotechnol. 2008. 18:1170–8.38). Kim HY., Kim HS. Upregulation of MIP-2 (CXCL2) expression by 15-deoxy-Delta(12,14)-prostaglandin J(2) in mouse peritoneal macrophages. Immunol Cell Biol. 2007. 85:60–7.39). Fu Y., Luo N., Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-delta 12, 14 prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002. 160:11–20.40). Tanabe S., Bodet C., Grenier D. Peptostreptococcus micros cell wall elicits a pro-inflammatory response in human macrophages. J Endotoxin Res. 2007. 13:219–26.
Article41). Chung J., Lee HS., Chung HY., Yoon TR., Kim HK. Salicylideneamino-2-thiophenol inhibits inflammatory mediator genes (RANTES. MCP-1, IL-8 and HIF-1alpha) expression induced by tert-butyl hydroperoxide via MAPK pathways in rat peritoneal macrophages. Biotechnol Lett. 2008. 30:1553–8.42). Sen U., Tyagi N., Patibandla PK., Dean WL., Tyagi SC., Roberts AM., Lominadze D. Fibrinogen-induced endothelin-1 production from endothelial cells. Am J Physiol Cell Physiol. 2009. 296:C840–7.
Article43). Natarajan R., Rosdahl J., Gonzales N., Bai W. Regulation of 12-lipoxygenase by cytokines in vascular smooth muscle cells. Hypertension. 1997. 30:873–9.
Article44). Preston IR., Hill NS., Warburton RR., Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2006. 290:L367–74.
Article45). Sasaki M., Hori MT., Hino T., Golub MS., Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens. 1997. 10:371–8.
Article46). Oyekan A., Balazy M., McGiff JC. Renal oxygenases: differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997. 273:R293–300.
Article47). Newaz MA., Oyekan AO. Contribution of renal oxygenases to glycerol-induced acute renal failure in the rat. J Cardiovasc Pharmacol. 2002. 39:834–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Lipopolysaccharide on the Expression of Chemokine Mig Gene in Mouse Peritoneal Macrophages
- Peptidoglycan Up-Regulates CXCL8 Expression via Multiple Pathways in Monocytes/Macrophages
- Effects of Capsaicin Pretreatment on the Functions of Mouse Peritoneal Macrophages
- Cytotoxicity of resident and lymphokine-activated mouse peritoneal macrophage against Trichomonas vaginalis
- Effects of interleukin-10 on chemokine KC gene expression by mouse peritoneal macrophages in response to Candida albicans