J Korean Surg Soc.
2009 Jun;76(6):337-347. 10.4174/jkss.2009.76.6.337.
Effect of Combination of Anticancer Agents and Nitroimidazoles on the Survival of Human Hepatocellular Carcinoma Cells under Hypoxic Conditions
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Catholic University of Daegu, Korea. leejw@cu.ac.kr
- 2Department of Surgery, College of Medicine, Catholic University of Daegu, Korea. shwpark@cu.ac.kr
- 3Department of Laboratory Medicine, College of Medicine, Catholic University of Daegu, Korea.
- 4Department of Pathology, College of Medicine, Catholic University of Daegu, Korea.
- 5Ansim Internal Medicine Clinic, Daegu, Korea.
- 6Department of Biochemistry, College of Medicine, Keimyung University, Daegu, Korea.
- KMID: 1464948
- DOI: http://doi.org/10.4174/jkss.2009.76.6.337
Abstract
-
PURPOSE: In a previous study, we have shown that anticancer agents inhibiting topoisomerases improve survival of tumor cells under hypoxic condition. In the present study, we evaluated whether and how cell survival effect of the anticancer agents under hypoxic conditions could be eliminated by the addition of nitroimidazoles, a class of bioreductive agents.
METHODS
Human hepatocellular carcinoma cells (HepG2) were incubated with different combinations of pimonidazole (1~1,000 microg/ml) and doxorubicin (0.1 or 1 microg/ml) concentrations under different O2 concentrations [1, 3, 5, 10 and 21 O2]. Then cell numbers, glucose concentrations and lactic acid concentrations in the medium were measured, and DNA fragmentation assay was performed. Finally, different combinations of nitroimidazoles, such as pimonidazole, misonidazole, etanidazole, tinidazole, metronidazole, ornidazole or dimetridazole, and anticancer agents, such as doxorubicin, campothecin, epirubicin, dactinomycin, etoposide or mitomycin C was added to the cell culture medium under hypoxic conditions (1% O2).
RESULTS
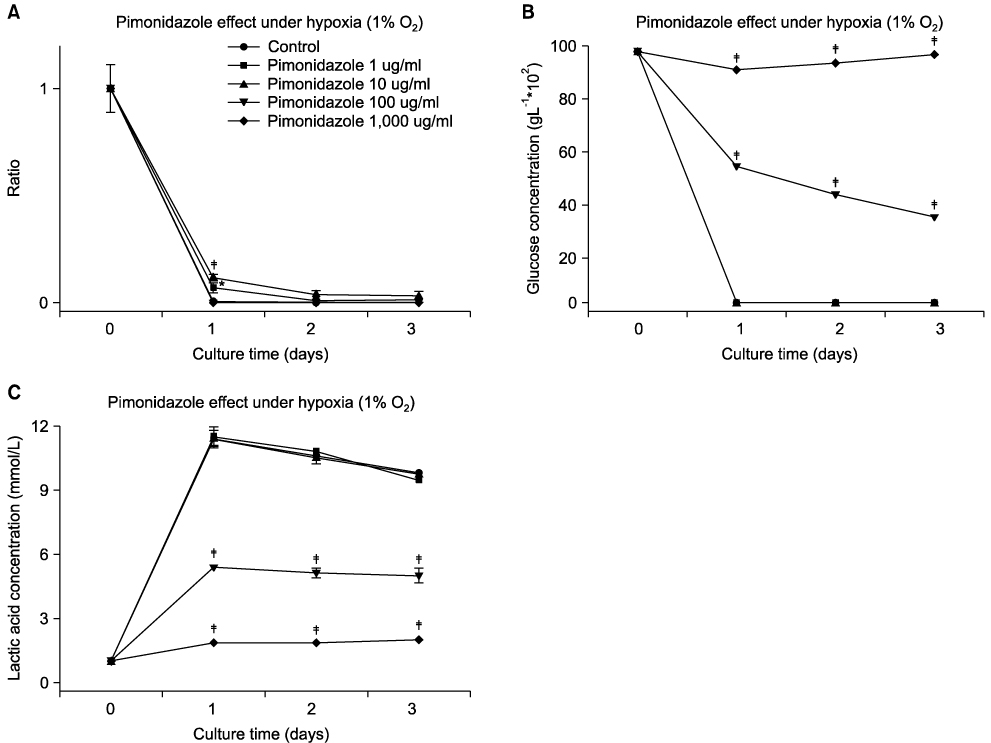
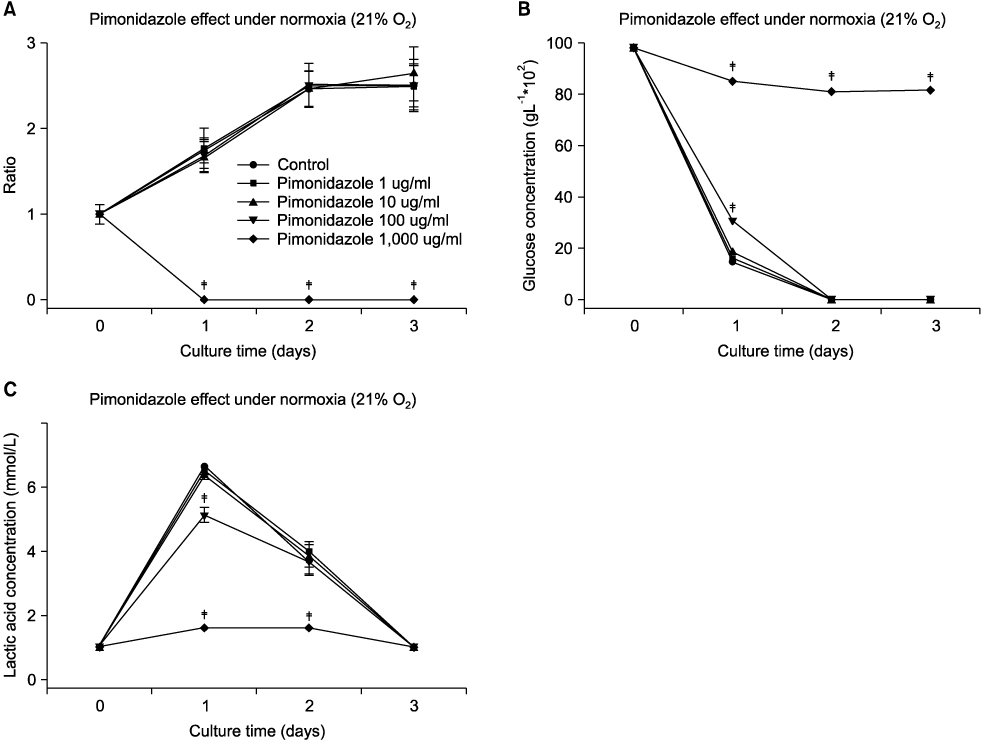
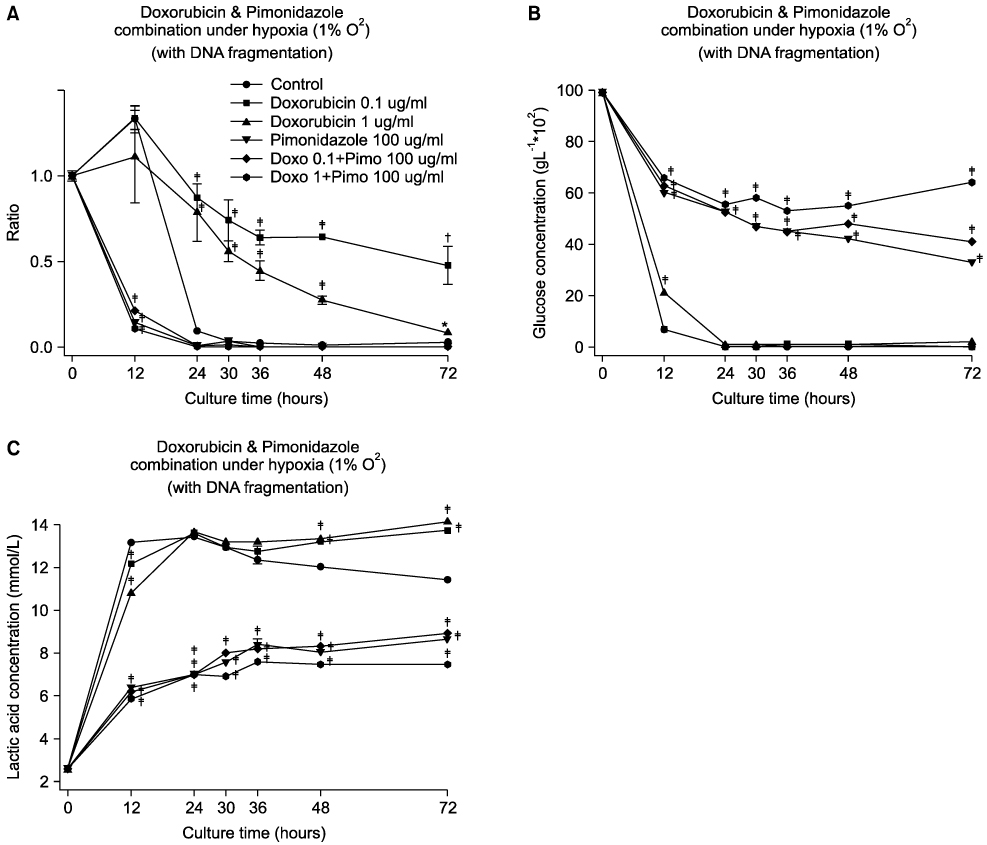
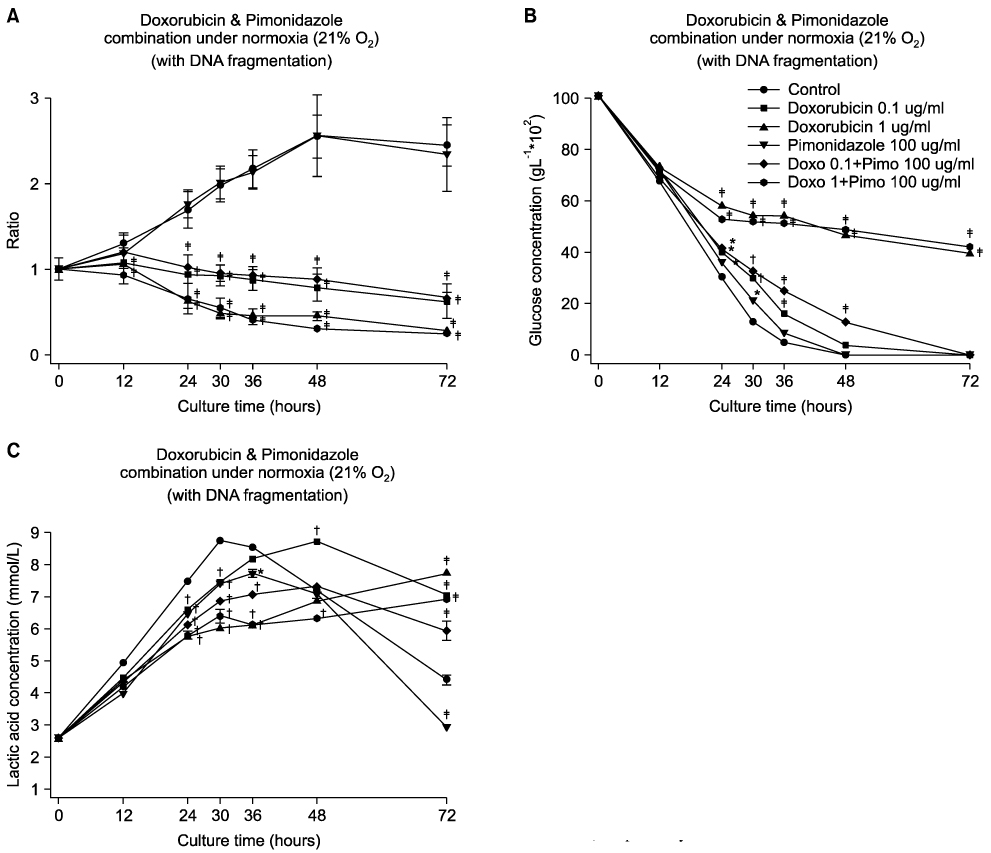
Pimonidazole at a concentration of 100 microg/ml eliminated cell survival effect of doxorubicin at the concentrations of 0.1 and 1 microg/ml under hypoxic condition (1% O2) by promoting apoptosis. Almost all the cells died even after 24 hours of incubation for all the oxygen concentrations at a combination of 100 microg/ml pimonidazole and 1 microg/ml doxorubicin. Finally, pimonidazole at a concentration of 100 microg/ml, and misonidazole or etanidazole at a concentration of 1,000 microg/ml eliminated cell survival effect of all the anticancer agents tested under hypoxic condition.
CONCLUSION
Combination therapy of doxorubicin (adriamycin) with pimonidazole can maximize dororubicin efficacy by eliminating cell survival effect of doxorubicin under hypoxic conditions in treating solid tumors, such as breast cancer.
MeSH Terms
-
Anoxia
Antineoplastic Agents
Apoptosis
Breast Neoplasms
Carcinoma, Hepatocellular
Cell Count
Cell Culture Techniques
Cell Survival
Dactinomycin
Dimetridazole
DNA Fragmentation
Doxorubicin
Epirubicin
Etanidazole
Etoposide
Glucose
Humans
Lactic Acid
Metronidazole
Misonidazole
Mitomycin
Nitroimidazoles
Ornidazole
Oxygen
Tinidazole
Antineoplastic Agents
Dactinomycin
Dimetridazole
Doxorubicin
Epirubicin
Etanidazole
Etoposide
Glucose
Lactic Acid
Metronidazole
Misonidazole
Mitomycin
Nitroimidazoles
Ornidazole
Oxygen
Tinidazole
Figure
Reference
-
1. Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med. 2007. 85:1301–1307.2. Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004. 9:Suppl 5. 4–9.3. Wouters A, Pauwels B, Lardon F, Vermorken JB. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist. 2007. 12:690–712.4. Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002. 8:878–884.5. Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007. 26:225–239.6. Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003. 94:1021–1028.7. Kurebayashi J, Otsuki T, Moriya T, Sonoo H. Hypoxia reduces hormone responsiveness of human breast cancer cells. Jpn J Cancer Res. 2001. 92:1093–1101.8. Lee YT, Han MJ, Lim SH, Park SH, Suh HS, Park JB, et al. Effect of antibiotics on the survival of human hepatocellular carcinoma cells under hypoxic conditions. J Korean Surg Soc. 2006. 71:31–38.9. Kim HS, Lee YM, Yoo MA, Lee JW, Ryu SH, Kim KW. Ofloxacin inhibits hypoxia/hypoglycemia-induced apoptosis in bovine aortic endothelial cells. J Korean Assoc Cancer Prev. 2001. 6:155–164.10. Lee JY, Lim SH, Park SH, Ahn KS, Suh HS, Lee J. Effect of antitumor agents on the survival of human hepatocellular carcinoma cells under hypoxic conditions. Korean J Med. 2007. 72:384–392.11. Denny WA. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000. 1:25–29.12. Tocher JH. Reductive activation of nitroheterocyclic compounds. Gen Pharmacol. 1997. 28:485–487.13. Teicher BA, Holden SA, al-Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990. 50:3339–3344.14. Brenner DE, Galloway S, Cooper J, Noone R, Hande KR. Improved high-performance liquid chromatography assay of doxorubicin: detection of circulating aglycones in human plasma and comparison with thin-layer chromatography. Cancer Chemother Pharmacol. 1985. 14:139–145.15. Muller C, Chatelut E, Gualano V, De Forni M, Huguet F, Attal M, et al. Cellular pharmacokinetics of doxorubicin in patients with chronic lymphocytic leukemia: comparison of bolus administration and continuous infusion. Cancer Chemother Pharmacol. 1993. 32:379–384.16. Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol). 2007. 19:397–417.17. Roberts JT, Bleehen NM, Workman P, Walton MI. A phase I study of the hypoxic cell radiosensitizer Ro-03-8799. Int J Radiat Oncol Biol Phys. 1984. 10:1755–1758.18. Saunders MI, Anderson PJ, Bennett MH, Dische S, Minchinton A, Stratford MR, et al. The clinical testing of Ro 03-8799--pharmacokinetics, toxicology, tissue and tumor concentrations. Int J Radiat Oncol Biol Phys. 1984. 10:1759–1763.19. A trial of Ro 03-8799 (pimonidazole) in carcinoma of the uterine cervix: an interim report from the Medical Research Council Working Party on advanced carcinoma of the cervix. Radiother Oncol. 1993. 26:93–103.20. Grigsby PW, Winter K, Wasserman TH, Marcial V, Rotman M, Cooper J, et al. Irradiation with or without misonidazole for patients with stages IIIB and IVA carcinoma of the cervix: final results of RTOG 80-05. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1999. 44:513–517.21. Chan P, Milosevic M, Fyles A, Carson J, Pintilie M, Rauth M, et al. A phase III randomized study of misonidazole plus radiation vs. radiation alone for cervix cancer. Radiother Oncol. 2004. 70:295–299.22. Drzymala RE, Wasserman TH, Won M, Shaw E, Cmelak AJ, Loeffler J, et al. A phase I-B trial of the radiosensitizer: etanidazole (SR-2508) with radiosurgery for the treatment of recurrent previously irradiated primary brain tumors or brain metastases (RTOG Study 95-02). Radiother Oncol. 2008. 87:89–92.23. Urtasun R, Feldstein ML, Partington J, Tanasichuk H, Miller JD, Russell DB, et al. Radiation and nitroimidazoles in supratentorial high grade gliomas: a second clinical trial. Br J Cancer. 1982. 46:101–108.24. Brezden CB, McClelland RA, Rauth AM. Mechanism of the selective hypoxic cytotoxicity of 1-methyl-2-nitroimidazole. Biochem Pharmacol. 1994. 48:361–370.25. Kuno Y, Shinomiya N. PR-000350, a novel hypoxic radiosensitizer, enhances tumor cell killing by promoting apoptosis preferentially in the S-phase fraction. Apoptosis. 2000. 5:69–77.26. Thomas CL. Taber's Cyclopedic Medical Dictionary. 1993. 17th ed. Philadelphia: F.A. Davis.27. Newman HF, Ward R, Workman P, Bleehen NM. The multi-dose clinical tolerance and pharmacokinetics of the combined radiosensitizers, Ro 03-8799 (pimonidazole) and SR 2508 (etanidazole). Int J Radiat Oncol Biol Phys. 1988. 15:1073–1083.28. Bleehen NM, Newman HF, Maughan TS, Workman P. A multiple dose study of the combined radiosensitizers Ro 03-8799 (pimonidazole) and SR 2508 (etanidazole). Int J Radiat Oncol Biol Phys. 1989. 16:1093–1096.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of antitumor agents on the survival of human hepatocellular carcinoma cells under hypoxic conditions
- Effect of Antibiotics on the Survival of Human Hepatocellular Carcinoma Cells under Hypoxic Conditions
- Effect of anticancer drugs and desferrioxamine in combination with radiation on hepatoma cell lines
- Everolimus Plus Ku0063794 Regimen Promotes Anticancer Effects against Hepatocellular Carcinoma Cells through the Paradoxical Inhibition of Autophagy
- CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells