Korean J Physiol Pharmacol.
2010 Feb;14(1):15-20. 10.4196/kjpp.2010.14.1.15.
Effect of Modulation of hnRNP L Levels on the Decay of bcl-2 mRNA in MCF-7 Cells
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. leejh@catholic.ac.kr
- 2Bioindustry Research Center, Korea Research Institute Bioscience and Biotechonology, Daejeon 305-806, Korea.
- KMID: 1457696
- DOI: http://doi.org/10.4196/kjpp.2010.14.1.15
Abstract
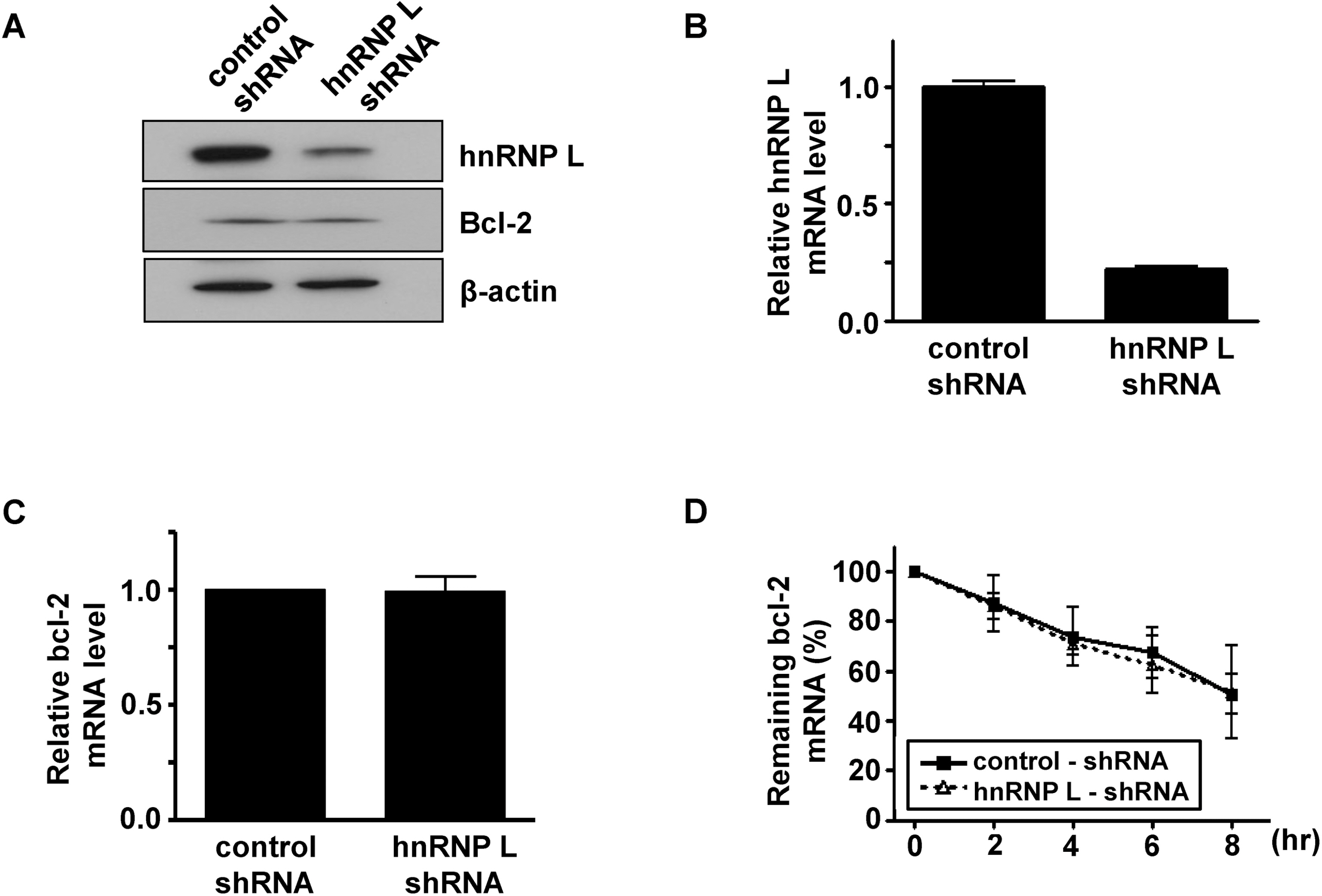
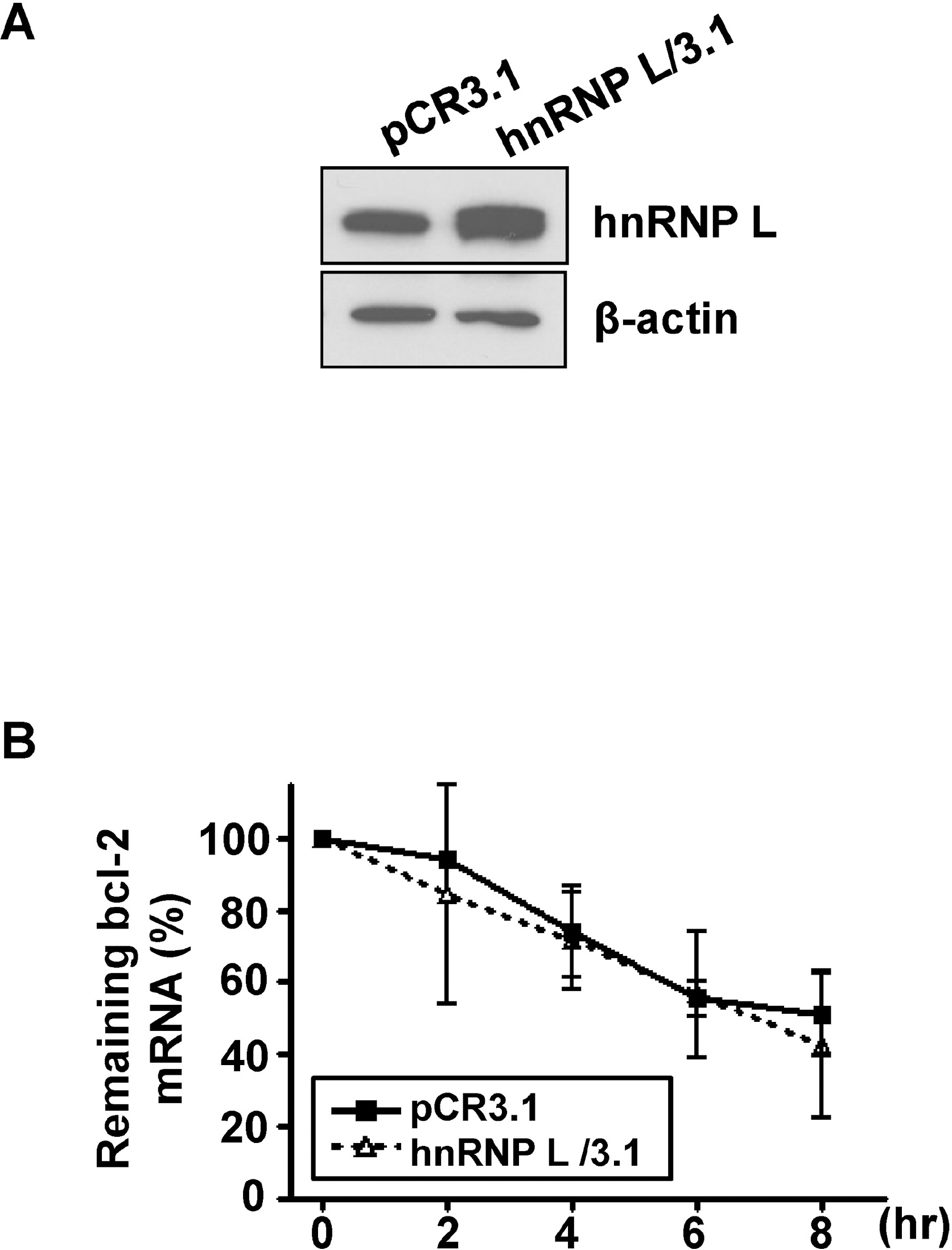
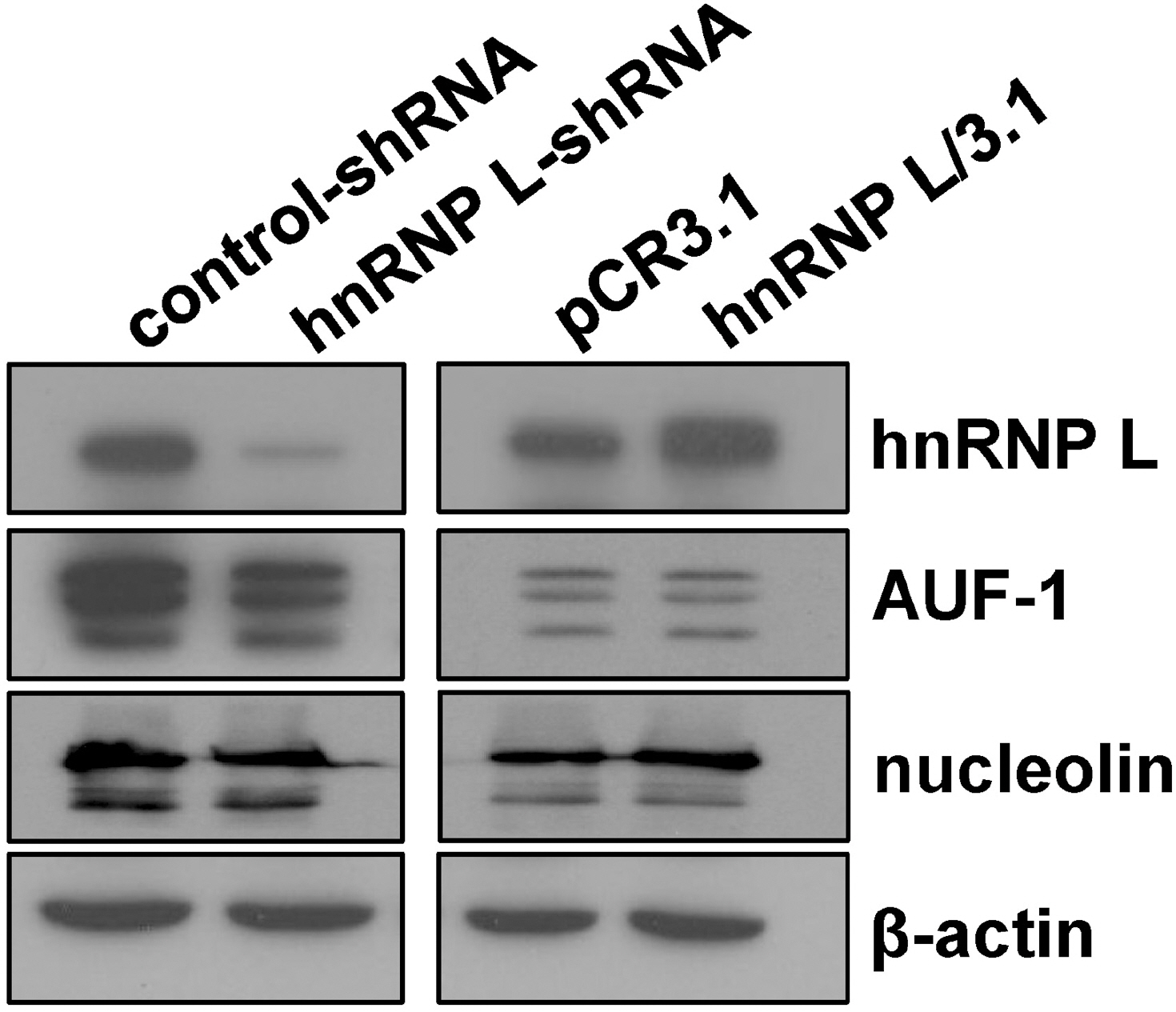
- It has been shown that CA repeats in the 3'-untranslated region (UTR) of bcl-2 mRNA contribute the constitutive decay of bcl-2 mRNA and that hnRNP L (heterogenous nuclear ribonucleoprotein L) interacts with CA repeats in the 3'-UTR of bcl-2 mRNA, both in vitro and in vivo. The aim of this study was to determine whether the alteration of hnRNP L affects the stability of bcl-2 mRNA in vivo. Human breast carcinoma MCF-7 cells were transfected with hnRNP L-specific shRNA or hnRNP L-expressing vector to decrease or increase hnRNP L levels, respectively, followed by an actinomycin D chase. An RT-PCR analysis showed that the rate of degradation of endogenous bcl-2 mRNA was not affected by the decrease or increase in the hnRNP L levels. Furthermore, during apoptosis or autophagy, in which bcl-2 expression has been reported to decrease, no difference in the degradation of bcl-2 mRNA was observed between control and hnRNP L-knock down MCF-7 Cells. On the other hand, the levels of AUF-1 and nucleolin, transacting factors for ARE in the 3'UTR of bcl-2 mRNA, were not significantly affected by the decrease in hnRNP L, suggesting that a disturbance in the quantitative balance between these transacting factors is not likely to interfere with the effect of hnRNP L. Collectively, the findings indicate that the decay of bcl-2 mRNA does not appear to be directly controlled by hnRNP L in vivo.
Keyword
MeSH Terms
-
3' Untranslated Regions
Apoptosis
Autophagy
Breast
Dactinomycin
Hand
Heterogeneous-Nuclear Ribonucleoprotein L
Heterogeneous-Nuclear Ribonucleoproteins
Humans
MCF-7 Cells
Phosphoproteins
Ribonucleoproteins
RNA, Messenger
RNA, Small Interfering
RNA-Binding Proteins
3' Untranslated Regions
Dactinomycin
Heterogeneous-Nuclear Ribonucleoprotein L
Heterogeneous-Nuclear Ribonucleoproteins
Phosphoproteins
RNA, Messenger
RNA, Small Interfering
RNA-Binding Proteins
Ribonucleoproteins
Figure
Reference
-
References
1. Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008; 27:6419–6433.
Article2. Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994; 124:1–6.
Article3. Davies AM. The Bcl-2 family of proteins, and the regulation of neuronal survival. Trends Neurosci. 1995; 18:355–358.
Article4. Greenlund LJ, Korsmeyer SJ, Johnson EM Jr. Role of BCL-2 in the survival and function of developing and mature sympathetic neurons. Neuron. 1995; 15:649–661.
Article5. Young RL, Korsmeyer SJ. A negative regulatory element in the bcl-2 5′-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993; 13:3686–3697.
Article6. Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000; 20:2890–2901.7. Riordan FA, Foroni L, Hoffbrand AV, Mehta AB, Wickremasinghe RG. Okadaic acid-induced apoptosis of HL60 leukemia cells is preceded by destabilization of bcl-2 mRNA and down-regulation of bcl-2 protein. FEBS Lett. 1998; 435:195–198.
Article8. Bandyopadhyay S, Sengupta TK, Fernandes DJ, Spicer EK. Taxol- and okadaic acid-induced destabilization of bcl-2 mRNA is associated with decreased binding of proteins to a bcl-2 instability element. Biochem Pharmacol. 2003; 66:1151–1162.
Article9. Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001; 265:11–23.
Article10. Schiavone N, Rosini P, Quattrone A, Donnini M, Lapucci A, Citti L, Bevilacqua A, Nicolin A, Capaccioli S. A conserved AU-rich element in the 3′ untranslated region of bcl-2 mRNA is endowed with a destabilizing function that is involved in bcl-2 down-regulation during apoptosis. FASEB J. 2000; 14:174–184.11. Donnini M, Lapucci A, Papucci L, Witort E, Tempestini A, Brewer G, Bevilacqua A, Nicolin A, Capaccioli S, Schiavone N. Apoptosis is associated with modifications of bcl-2 mRNA AU-binding proteins. Biochem Biophys Res Commun. 2001; 287:1063–1069.
Article12. Lapucci A, Donnini M, Papucci L, Witort E, Tempestini A, Bevilacqua A, Nicolin A, Brewer G, Schiavone N, Capaccioli S. AUF-1 Is a bcl-2 A + U-rich element-binding protein involved in bcl-2 mRNA destabilization during apoptosis. J Biol Chem. 2002; 277:16139–16146.
Article13. Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem. 2004; 279:10855–10863.
Article14. Donnini M, Lapucci A, Papucci L, Witort E, Jacquier A, Brewer G, Nicolin A, Capaccioli S, Schiavone N. Identification of TINO: a new evolutionarily conserved BCL-2 AU-rich element RNA-binding protein. J Biol Chem. 2004; 279:20154–20166.15. Lee JH, Jeon MH, Seo YJ, Lee YJ, Ko JH, Tsujimoto Y, Lee JH. CA repeats in the 3′-untranslated region of bcl-2 mRNA mediate constitutive decay of bcl-2 mRNA. J Biol Chem. 2004; 279:42758–42764.
Article16. Lee DH, Lim MH, Youn DY, Jung SE, Ahn YS, Tsujimoto Y, Lee JH. hnRNP L binds to CA repeats in the 3'UTR of bcl-2 mRNA. Biochem Biophys Res Commun. 2009; 382:583–587.
Article17. Park HG, Yoon JY, Choi M. Heterogeneous nuclear ribonucleo-protein D/AUF1 interacts with heterogeneous nuclear ribonucleoprotein L. J Biosci. 2007; 32:1263–1272.
Article18. Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994; 9:1799–1805.19. Suzuki A, Matsuzawa A, Iguchi T. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene. 1996; 13:31–37.20. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005; 122:927–939.
Article21. Hui J, Reither G, Bindereif A. Novel functional role of CA repeats and hnRNP L in RNA stability. RNA. 2003; 9:931–936.
Article22. Hui J, Hung LH, Heiner M, Schreiner S, Neumüller N, Reither G, Haas SA, Bindereif A. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J. 2005; 24:1988–1998.
Article23. Rothrock CR, House AE, Lynch KW. HnRNP L represses exon splicing via a regulated exonic splicing silencer. EMBO J. 2005; 24:2792–2802.
Article24. Shih SC, Claffey KP. Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. J Biol Chem. 1995; 274:1359–1365.
Article25. Söderberg M, Raffalli-Mathieu F, Lang MA. Identification of a regulatory cis-element within the 3′-untranslated region of the murine inducible nitric oxide synthase (iNOS) mRNA; interaction with heterogeneous nuclear ribonucleoproteins I and L and role in the iNOS gene expression. Mol Immunol. 2007; 44:434–442.
Article26. Hamilton BJ, Nichols RC, Tsukamoto H, Boado RJ, Pardridge WM, Rigby WF. hnRNP A2 and hnRNP L bind the 3'UTR of glucose transporter 1 mRNA and exist as a complex in vivo. Biochem Biophys Res Commun. 1999; 261:646–651.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines
- The Role of bcl-2 and p53 in Tamoxifen-Induced Apoptosis of Human Breast Cancer Cell Lines
- Aspirin-induced Bcl-2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells
- Inductoin of Radioresistance by Overexpression of Glutathione S-Transferase K1 (hGSTK1) in MCF-7 Cells
- Effects of HMGB-1 Overexpression on Cell-Cycle Progression in MCF-7 Cells