J Breast Cancer.
2009 Jun;12(2):85-91. 10.4048/jbc.2009.12.2.85.
Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 2Department of Surgery, Ewha Womans University School of Medicine, Ewha Medical Research Institute, Seoul, Korea. mbit@mm.ewha.ac.kr
- KMID: 2175202
- DOI: http://doi.org/10.4048/jbc.2009.12.2.85
Abstract
-
PURPOSE: It has been demonstrated ellagic acid can inhibit tumor growth. However, the mechanism that elicits the anti-proliferative effect of ellagic acid is poorly understood. Our objective in this study was to evaluate the biological activity of ellagic acid by comparing the anti-proliferative effect and the apoptotic pathway of ellagic acid between the 2 human breast cancer cell lines.
METHODS
The MCF-7 and MDA-MB-231 human breast cancer cell lines were used as cell models. The anti-proliferstive effect was evaluated by using a MTT assay. The cell cycle was analyzed by flow cytometry. Western blotting was performed to show the expressions of bcl-xL, cytochrome c, surviving, c-fos and pS2.
RESULTS
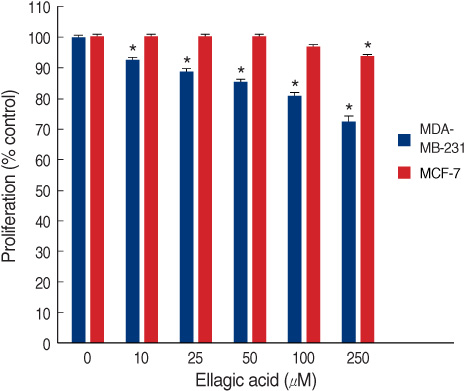
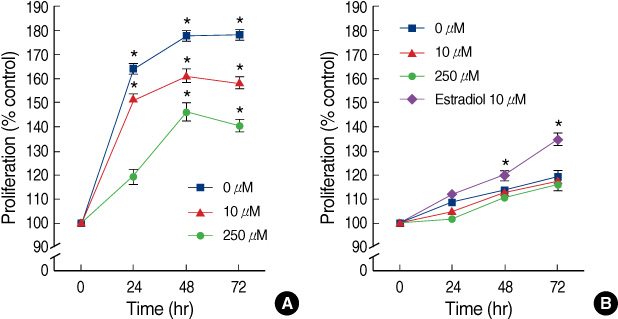
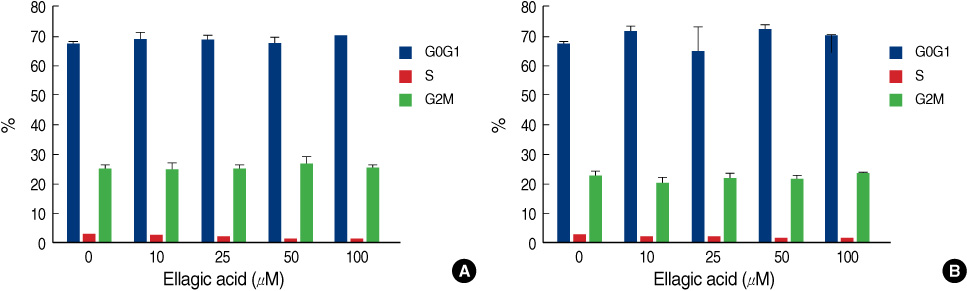
The ellagic acid in the MDA-MB-231 cells showed significant anti-proliferative effects with dose dependent pattern. The anti-proliferative effects in MCF-7 cells were observed in only at a high concentration. Ellagic acid had no effect on the cell cycle in both breast cancer cells. In MDA-MB-231, the expression of bcl-xL was decreased with the decreasing concentration of ellagic acid. The expression of cytochrome c in the cytosol was increased with the decreased expression of bcl-xL. Ellagic acid also decreased the expression of survivin. In the MCF-7 cells, the expressions of bcl-xL and cytochrome c showed no change after treatment with ellagic acid even at a high dose. Ellagic acid was able to induce an up-regulation of c-fos and pS2 protein in MCF-7.
CONCLUSION
Ellagic acid has an anti-proliferative effect in the MDA-MB-231 cells. This effect of ellagic acid is through the intrinsic pathway in MDA-MB-231 cells. However, the expression of bcl-xL showed no change in the MCF-7 cells. Ellagic acid has a different anti-proliferative effect between the two human breast cancer cell lines.
Keyword
MeSH Terms
Figure
Reference
-
1. Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004. 134:613–617.
Article2. Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica Granatum) for human breast cancer. Breast Cancer Res Treat. 2002. 71:203–217.
Article3. Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006. 54:980–985.
Article4. Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005. 16:360–367.
Article5. Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999. 129:758S–767S.
Article6. Papoutsi Z, Kassi E, Tsiapara A, Fokialakis N, Chrousos GP, Moutsatsou P. Evaluation of estrogenic/antiestrogenic activity of ellagic acid via the estrogen receptor subtypes ERalpha and ERbeta. J Agric Food Chem. 2005. 53:7715–7720.
Article7. Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004. 1030:434–441.
Article8. Saleem A, Husheem M, Harkonen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of terminalia chebula retz. fruit. J Ethnopharmacol. 2002. 81:327–336.
Article9. Li TM, Chen GW, Su CC, Lin JG, Yeh CC, Cheng KC, et al. Ellagic acid induced p53/p21 expression, G1 arrest and apoptosis in human bladder cancer T24 cells. Anticancer Res. 2005. 25:971–979.10. Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005. 218:141–151.
Article11. Smith WA, Freeman JW, Gupta RC. Effect of chemopreventive agents on DNA adduction induced by the potent mammary carcinogen dibenzo[a,l]pyrene in the human breast cells MCF-7. Mutat Res. 2001. 480-481:97–108.
Article12. Maciel ME, Castro GD, Castro JA. Inhibition of the rat breast cytosolic bioactivation of ethanol to acetaldehyde by some plant polyphenols and folic acid. Nutr Cancer. 2004. 49:94–99.
Article13. Losso JN, Bansode RR, Trappey A 2nd, Bawadi HA, Truax R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004. 15:672–678.
Article14. Labrecque L, Lamy S, Chapus A, Mihoubi S, Durocher Y, Cass B, et al. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis. 2005. 26:821–826.
Article15. Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006. 17:611–625.
Article16. Bowes T, Singh B, Gupta RS. Subcellular localization of fumarase in mammalian cells and tissues. Histochem Cell Biol. 2007. 127:335–346.
Article17. Tondera D, Santel A, Schwarzer R, Dames S, Giese K, Klippel A, et al. Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J Biol Chem. 2004. 279:31544–31555.
Article18. Stearns V, Coop A, Singh B, Gallagher A, Yamauchi H, Lieberman R, et al. A pilot surrogate end point biomarker trial of perillyl alcohol in breast neoplasia. Clin Cancer Res. 2004. 10:7583–7591.
Article19. Wagner JE, Huff JL, Rust WL, Kingsley K, Plopper GE. Perillyl alcohol inhibits breast cell migration without affecting cell adhesion. J Biomed Biotechnol. 2002. 2:136–140.
Article20. Nhan S, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr Cancer. 2005. 53:73–81.
Article21. Nikander E, Metsa-Heikkila M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003. 88:5180–5185.
Article22. Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006. 54:1611–1620.
Article23. Narayanan BA, Re GG. IGF-II down regulation associated cell cycle arrest in colon cancer cells exposed to phenolic antioxidant ellagic acid. Anticancer Res. 2001. 21:359–364.24. Kuo PL, Hsu YL, Kuo YC, Chang CH, Lin CC. The anti-proliferative inhibition of ellipticine in human breast MDA-MB-231 Cancer cells is through cell cycle arrest and apoptosis induction. Anticancer Drugs. 2005. 16:789–795.
Article25. Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006. 5:2931–2945.
Article26. Ka H, Hunt JS. Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas. Am J Pathol. 2003. 163:413–422.
Article27. Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998. 58:5315–5320.28. Li QX, Zhao J, Liu JY, Jia LT, Huang HY, Xu YM, et al. Survivin stable knockdown by siRNA inhibits tumor cell growth and angiogenesis in breast and cervical cancers. Cancer Biol Ther. 2006. 5:860–866.
Article29. Rumjahn SM, Javed MA, Wong N, Law WE, Buxton IL. Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. Br J Cancer. 2007. 97:1372–1380.
Article30. Brown AM, Jeltsch JM, Roberts M, Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci USA. 1984. 81:6344–6348.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Studies on the Polyarnine Involvement in MCF - 7 and MDA - MB - 231 Breast Cancer Cell Proliferation
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways
- Effect of corosolic acid on apoptosis and angiogenesis in MDA-MB-231 human breast cancer cells
- Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells