J Korean Soc Spine Surg.
2011 Mar;18(1):1-12. 10.4184/jkss.2011.18.1.1.
Variations of Neurotrophic Factors and It's Importances in Spinal Cord Injured Rats and Beagle Dogs
- Affiliations
-
- 1Department of Orthopaedic Surgery, Chungnam National University School of Medicine, Research Institute of Medical Science, Daejeon, Korea. jyyang@cnu.ac.kr
- 2Department of Orthopaedic Surgery, Sun General Hospital, Daejeon, Korea.
- KMID: 1448025
- DOI: http://doi.org/10.4184/jkss.2011.18.1.1
Abstract
- STUDY DESIGN: Experimental, prospective study
OBJECTIVES
To examine the changes in the variable factors after an acute spinal cord injury(SCI) in rats and dogs simultaneously. SUMMARY OF LITERATURE REVIEW: No study has examined the variations of several factors in a SCI model in different species.
MATERIALS AND METHODS
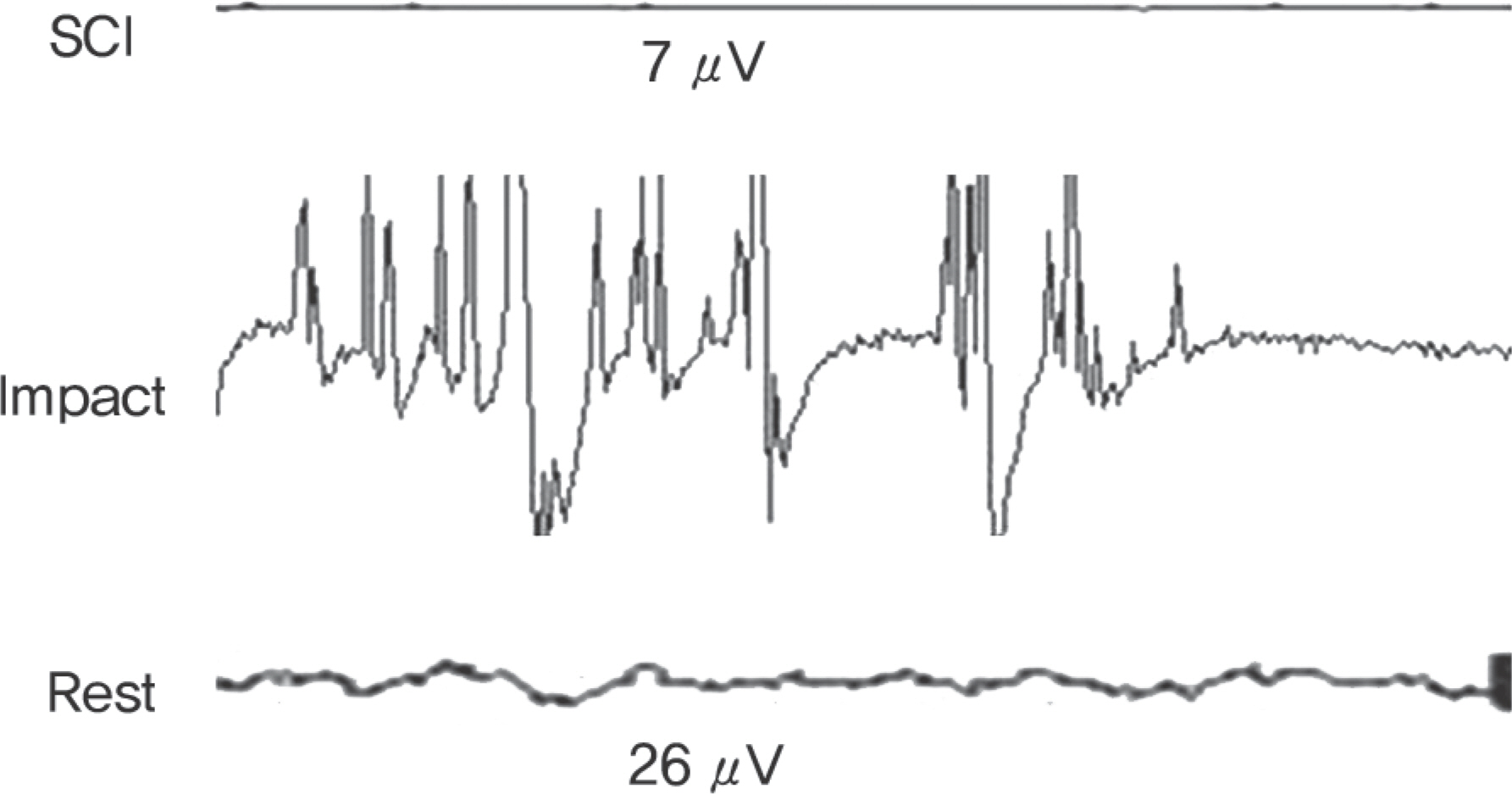
In rats, a laminectomy was performed at the T10 level and the injured spinal cord was extracted. In Beagle dogs, the laminectomy level was T10 and T11. The motor function was evaluated using a modified Tarlov's scale. A RT2 profiler PCR array was used to examine each factor (inflammatory cytokines, factors-related with apoptosis, neurotrophic factors, factors-related with extraceullar matrix).
RESULTS
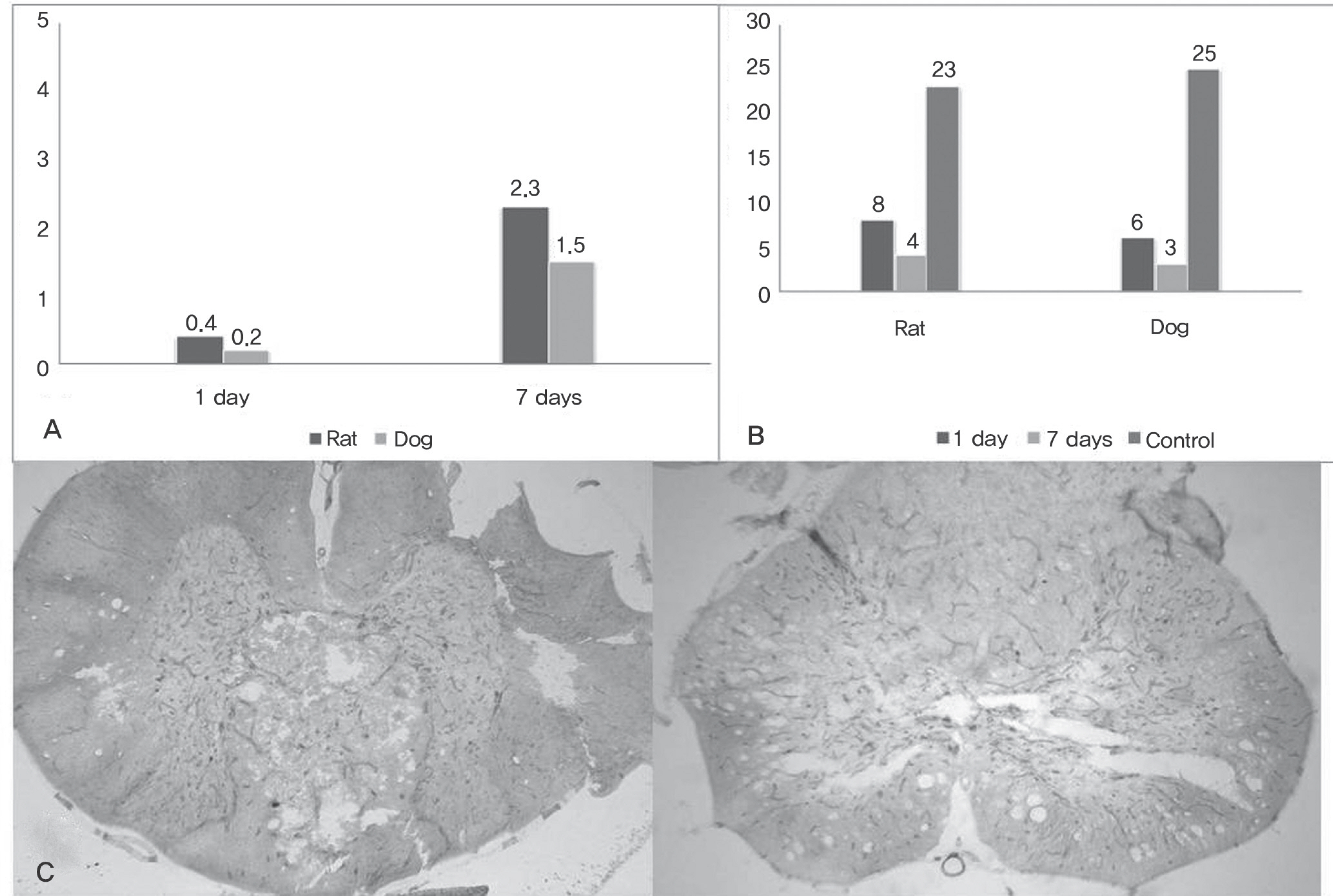
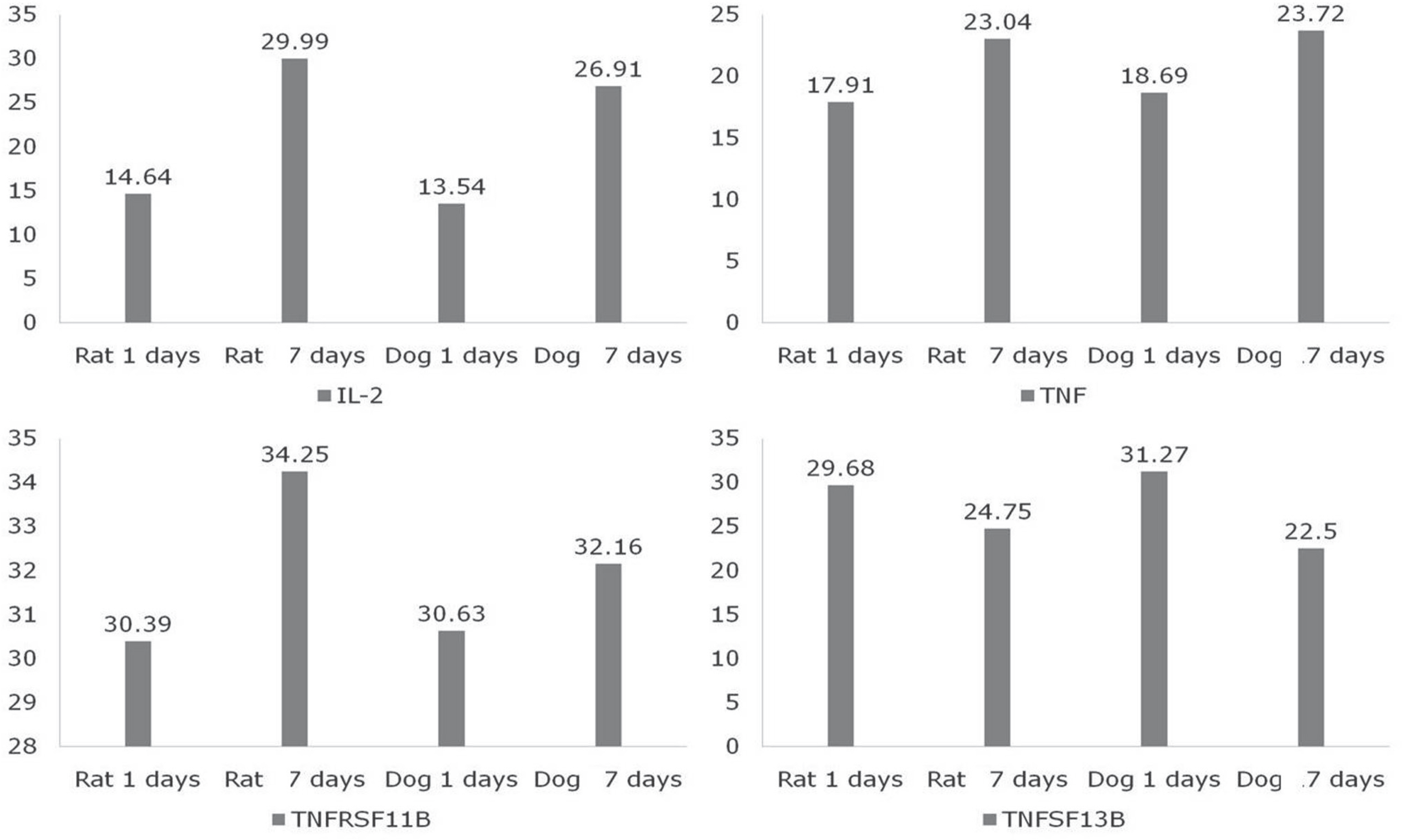
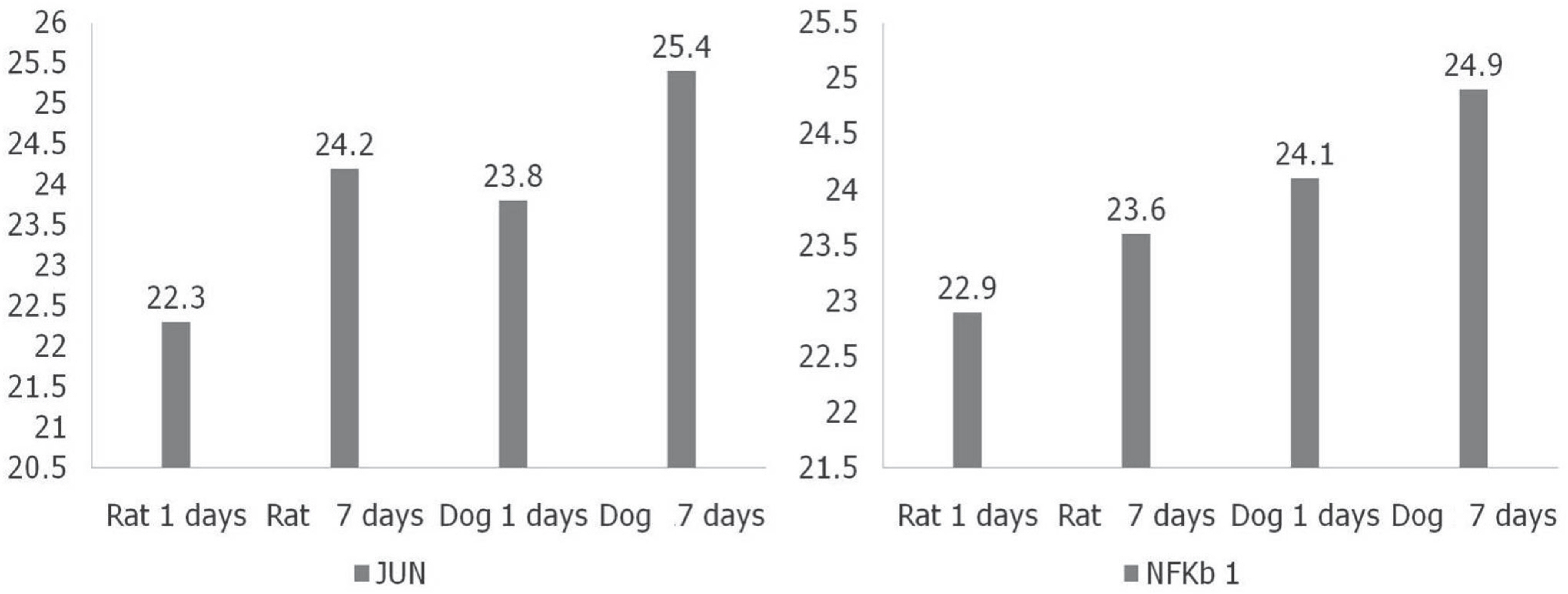
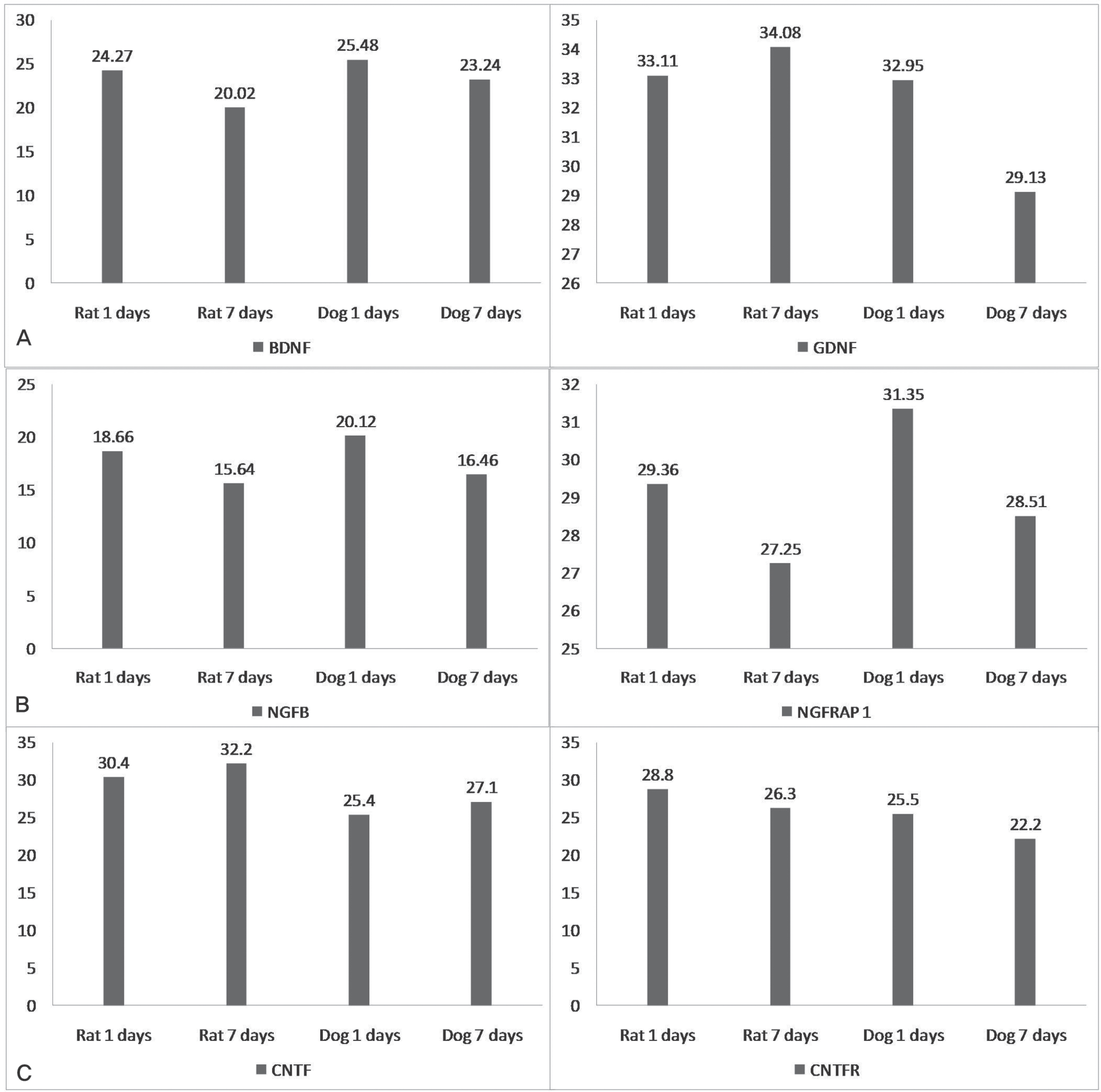
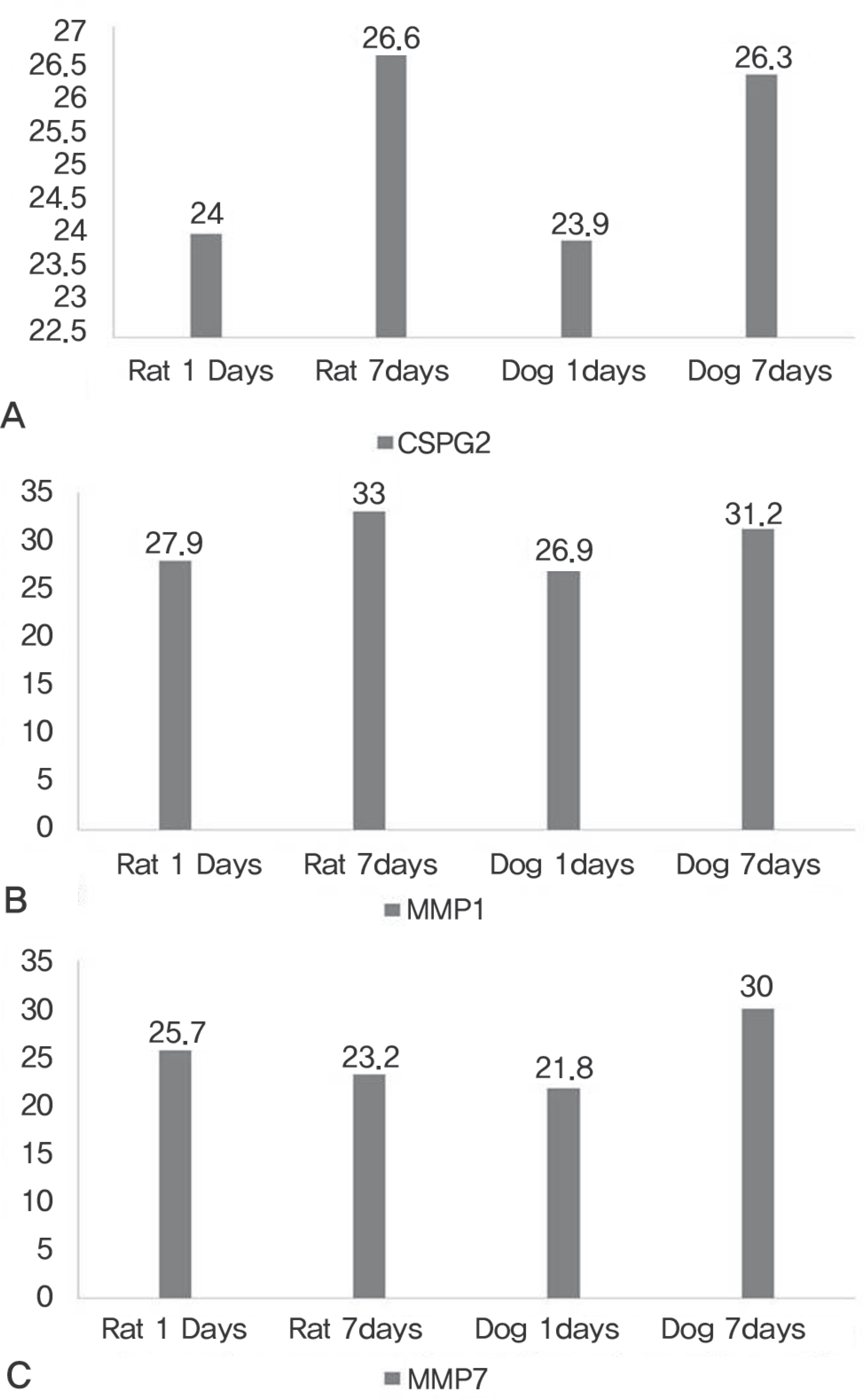
IL-2, TNF, TNFRSF11B increased with time and showed no statistical difference between two species, but TNFSF13B showed a significant difference. BDNF decreased with time in both species, and GDNF was significantly lower in dogs. NGFbeta, CTNF and its receptors showed no significant changes in the two species. MMP1 increased in both species but MMP7 decreased in rats and increased in dogs with time, and showed a significant difference between species.
CONCLUSION
The change in inflammatory cytokines and extracellular matrix correlates with each factor in the combined patterns. Moreover, during the first week after SCI, inflammatory cytokines, apoptosis, neutrophic factors, and extracellular matrix factors may show a partial difference between experimental animals, which means that an animal model can be selected according to the particular experimental plan.
Keyword
MeSH Terms
-
Animals
Apoptosis
Brain-Derived Neurotrophic Factor
Cytokines
Dogs
Extracellular Matrix
Glial Cell Line-Derived Neurotrophic Factor
Interleukin-2
Laminectomy
Models, Animal
Nerve Growth Factors
Polymerase Chain Reaction
Prospective Studies
Rats
Spinal Cord
Spinal Cord Injuries
Brain-Derived Neurotrophic Factor
Cytokines
Glial Cell Line-Derived Neurotrophic Factor
Interleukin-2
Nerve Growth Factors
Figure
Reference
-
1. Lee JK. Pathophysiology of acute spinal cord injury. J Korean Soc Spine Surg. 2009; 16:64–70.
Article2. You JW, Sohn HM. Recent advances in the pathophysiology and treatment of acute spinal cord injury. J Korean Soc Spine Surg. 2008; 15:204–13.3. Fernandez E, Pallini R, Marchese E, et al. Experimental studies on spinal cord injuries in the last fifteen years. Neurol Res. 1991; 13:138–59.
Article4. West NR, Leblanc V, Collins GH. Support of axonal regrowth by endogenous mechanisms following spinal cord injury in adult rats. Neuropathology. 2001; 21(3):188–202.
Article5. Yang JY, Lee HH, Lee JK. The Impact of Combined Treatment with Aminoguanidine and Methylprednisolone on Neurological Recovery after Spinal Cord Injury. J Korean Orthop Assoc. 2007; 42:444–52.6. Becker D, Sadowsky CL, Mcdonald JW. Restoring function after spinal cord injury. Neurologist. 2003; 9:1–15.
Article7. Gonç alves L, Silva R, Pinto-Ribeiro F, et al. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008; 213:48–56.8. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997; 3:73–6.
Article9. Akassoglou K, Bauer J, Kassitotis G, et al. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: Models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998; 153:801–13.10. Yang JY, Lee JK, Kim KT, Lee HJ, Byun BN, Ahn SH. The Expression and Function of the Tumor Necrosis Factor Receptor I (TNFRI), TNFRII, and X-Linked Inhibitor of Apoptosis Genes after Spinal Cord Injury in Rats. J Korean Soc Spine Surg. 2004; 11:14–23.
Article11. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998; 17:3261–70.
Article12. Johnson Jr EM, Greenlund LJ, Akins PT, Hsu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995; 12:843–52.13. Olson L. Medicine: clearing a path for nerve growth. Nature. 2002; 416:589–90.14. Conover JC, Erickson JT, Katz DM, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995; 375:235–8.
Article15. Coumans JV, Lin TT, Dai HN, et al. Axonal regenration and functional recovery after complete spinal cord transection in rats by delayes treatment with transplants and neurotrophins. J Neurosci. 2001; 21:9334–44.16. Davies AM. Role of neurotrophins in the developing nervous system. J Neurobiol. 1994; 25:1334–48.17. Hofer MM, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988; 331:261–2.
Article18. Baker-Herman TL, Fuller DD, Bavis RW, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature Neurosci. 2004; 7:48–55.
Article19. Lawrence JM, Keegan DJ, Muir EM, et al. Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 2004; 45:267–74.
Article20. Longo FM, Holtzman DM, Grimes ML, Mobley WC. Nerve growth factor: actions in the peripheral and central nervous systems. Fallon J, Loughlin S, editors. eds.Neurotrophic factors. New York: Academic Press;1993. 209-56.
Article21. Arenas E, Trupp M, Akerud P, Ibanez CF. GDNF prevents degeneration and promotes the phenotype of brain norad-renergic neurons in vivo. Neuron. 1995; 15:1465–73.
Article22. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain do-paminergic neurons. Science. 1993; 260:1130–2.
Article23. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001; 294:1945–8.
Article24. DeChiara TM, Vejsada R, Poueymirou WT, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995; 83:313–22.
Article25. Sleeman MW, Anderson KD, Lambert PD, et al. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Phar-maceutica acta Helvetiae. 2000; 74:265–72.26. Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007; 17:120–7.
Article27. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999; 49:377–91.
Article28. Sandvig A, Berry M, Barett LB, Butt A, Logan A. Myelin-, reactive glia-, and scarderived CNS axon growth inhibi-tors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004; 46:225–51.
Article29. Hong CH, Yang JY, Lee JK, Song HS. The Effect of Methylprednisolone and Riluzole on Axonal Growth after Acute Spinal Cord Injury in Rats. J Korean Orthop Assoc. 2008; 43:783–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats
- Changes of BDNF (Brain Derived Neurotrophic Factor) Expression Associated with Urodynamic Changes in Rat Spinal Cord Injury Animal Model
- The effect of 4-Methylcatechol treatment in chronic constrictive injury of the rat sciatic nerve on the allodynia and spinal neurotrophic factors
- Fracture of Femur Neck with Heterotopic Ossification in Spinal Cord Injured Patient
- Increased CNTF Expression in the Reactive Astrocyte Following Spinal Cord Injury in Rats