J Vet Sci.
2016 Mar;17(1):97-102. 10.4142/jvs.2016.17.1.97.
Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea. hykim@konkuk.ac.kr
- 2Department of Veterinary Surgery, College of Veterinary Medicine, Gyeongsang National University, Jinju 52828, Korea.
- 3Seoul Cord Blood Bank, Histostem Co, Seoul 05372, Korea.
- 4Department of Veterinary Clinical Pathology, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 5Avian Disease Laboratory, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- KMID: 2363340
- DOI: http://doi.org/10.4142/jvs.2016.17.1.97
Abstract
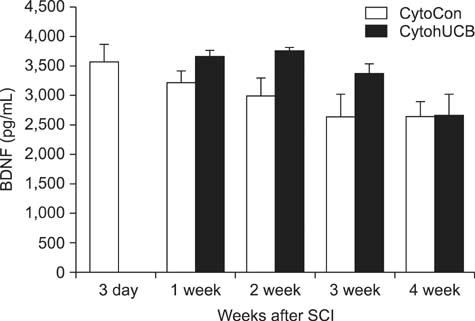
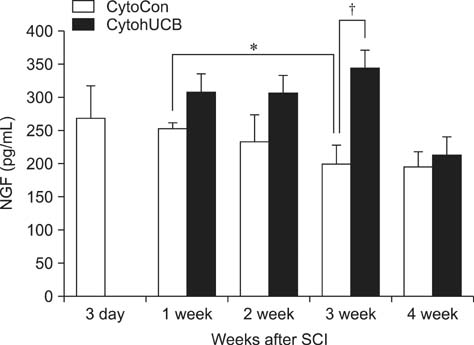
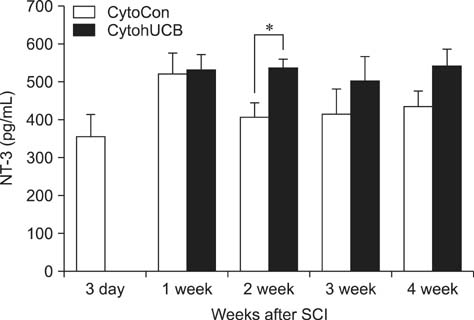
- We induced percutaneous spinal cord injuries (SCI) using a balloon catheter in 45 rats and transplanted human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) at the injury site. Locomotor function was significantly improved in hUCB-MSCs transplanted groups. Quantitative ELISA of extract from entire injured spinal cord showed increased expression of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and neurotrophin-3 (NT-3). Our results show that treatment of SCI with hUCB-MSCs can improve locomotor functions, and suggest that increased levels of BDNF, NGF and NT-3 in the injured spinal cord were the main therapeutic effect.
Keyword
MeSH Terms
-
Animals
Brain-Derived Neurotrophic Factor/*genetics
*Cord Blood Stem Cell Transplantation
Enzyme-Linked Immunosorbent Assay
Gene Expression Profiling
*Gene Expression Regulation
Humans
Locomotion
Nerve Growth Factor/genetics
Rats
Spinal Cord Injuries/*therapy
Brain-Derived Neurotrophic Factor
Nerve Growth Factor
Figure
Reference
-
1. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004; 35:2385–2389.
Article2. Bradbury EJ, King VR, Simmons LJ, Priestley JV, McMahon SB. NT-3, but not BDNF, prevents atrophy and death of axotomized spinal cord projection neurons. Eur J Neurosci. 1998; 10:3058–3068.
Article3. Chua SJ, Bielecki R, Yamanaka N, Fehlings MG, Rogers IM, Casper RF. The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine (Phila Pa 1976). 2010; 35:1520–1526.
Article4. Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, Han H, Lee JH, Choe BY, Lee SY, Kim HY. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res. 2009; 87:3554–3567.
Article5. Chung WH, Lee JH, Chung DJ, Yang WJ, Lee AJ, Choi CB, Chang HS, Kim DH, Chung HJ, Suh HJ, Hwang SH, Han H, Do SH, Kim HY. Improved rat spinal cord injury model using spinal cord compression by percutaneous method. J Vet Sci. 2013; 14:329–335.
Article6. Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, Rao JS, Dinh DH. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 2007; 24:391–410.
Article7. Domanska-Janik K, Buzanska L, Lukomska B. A novel, neural potential of non-hematopoietic human umbilical cord blood stem cells. Int J Dev Biol. 2008; 52:237–248.
Article8. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000; 109:235–242.
Article9. Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005; 380:322–325.
Article10. Franklin RJM, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997; 50:337–344.
Article11. Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2004; 321:102–108.
Article12. Ha Y, Yoon SH, Park HC, Kim KN, Yoon DH, Cho YE. Transplantation of human umbilical cord blood improves neurological outcomes in the rats after traumatic spinal cord injury. J Korean Neurosurg Soc. 2005; 35:302–308.13. Jeffery ND, Lakatos A, Franklin RJM. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma. 2005; 22:1282–1293.
Article14. Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005; 7:368–373.
Article15. Keirstead HS, Ben-Hur T, Rogister B, O'Leary MT, Dubois-Dalcq M, Blakemore WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999; 19:7529–7536.
Article16. Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005; 25:4694–4705.
Article17. Kim DH, Gutin PH, Noble LJ, Nathan D, Yu JS, Nockels RP. Treatment with genetically engineered fibroblasts producing NGF or BDNF can accelerate recovery from traumatic spinal cord injury in the adult rat. Neuroreport. 1996; 7:2221–2225.
Article18. Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010; 27:131–138.
Article19. Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien). 2005; 147:985–992.
Article20. Lee JH, Chang HS, Kang EH, Chung DJ, Choi CB, Lee JH, Hwang SH, Han H, Kim HY. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine. 2009; 11:749–757.
Article21. Lee JH, Chung WH, Kang EH, Chung DJ, Choi CB, Chang HS, Lee JH, Hwang SH, Han H, Choe BY, Kim HY. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J Neurol Sci. 2011; 300:86–96.
Article22. Li XL, Zhang W, Zhou X, Wang XY, Zhang HT, Qin DX, Zhang H, Li Q, Li M, Wang TH. Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides. 2007; 41:135–143.
Article23. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007; 8:275–282.
Article24. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004; 21:33–39.
Article25. McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999; 5:1410–1412.
Article26. Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000; 17:1219–1231.
Article27. Qin DX, Zou XL, Luo W, Zhang W, Zhang HT, Li XL, Zhang H, Wang XY, Wang TH. Expression of some neurotrophins in the spinal motoneurons after cord hemisection in adult rats. Neurosci Lett. 2006; 410:222–227.
Article28. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008; 25:E2.
Article29. Sanberg PR, Willing AE, Garbuzova-Davis S, Saporta S, Liu G, Sanberg CD, Bickford PC, Klasko SK, El-Badri NS. Umbilical cord blood derived stem cells and brain repair. Ann N Y Acad Sci. 2005; 1049:67–83.30. Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994; 367:170–173.
Article31. Schultz SS. Adult stem cell application in spinal cord injury. Curr Drug Targets. 2005; 6:63–73.
Article32. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine(Phila Pa 1976). 2001; 26(Suppl 24):S2–S12.
Article33. Shang AJ, Hong SQ, Xu Q, Wang HY, Yang Y, Wang ZF, Xu BN, Jiang XD, Xu RX. NT-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011; 19:102–113.
Article34. Tetzlaff W, Kobayashi NR, Giehl KM, Tsui BJ, Cassar SL, Bedard AM. Response of rubrospinal and corticospinal neurons to injury and neurotrophins. Prog Brain Res. 1994; 103:271–286.35. Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci Lett. 2006; 398:129–134.
Article36. Yang HJ, Yang XY, Ba YC, Pang JX, Meng BL, Lin N, Li LY, Dong XY, Zhao Y, Tian CF, Wang TH. Role of neurotrophin 3 in spinal neuroplasticity in rats subjected to cord transection. Growth Factors. 2009; 27:237–246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Umbilical Cord Blood Transplantation
- Functional Recovery in Complete Spinal Cord Injury after Transplantation of Human Umbilical Cord Blood Cells in Rats
- Transplantation of Human Umbilical Cord Blood Improves Neurological Outcomes in the Rats after Traumatic Spinal Cord Injury
- Umbilical Cord Blood Stem Cell Transplantation