Korean J Physiol Pharmacol.
2013 Feb;17(1):23-30. 10.4196/kjpp.2013.17.1.23.
Comparison of Ectopic Gene Expression Methods in Rat Neural Stem Cells
- Affiliations
-
- 1Laboratory of Stem Cell and Molecular Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. hyunjungkim@cau.ac.kr
- KMID: 1432759
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.23
Abstract
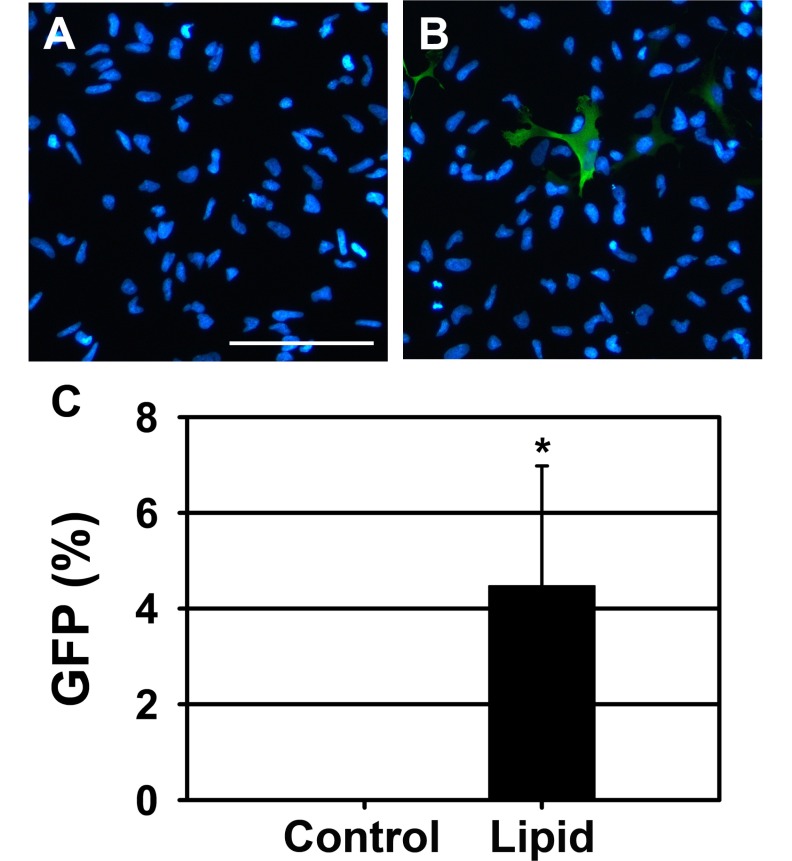
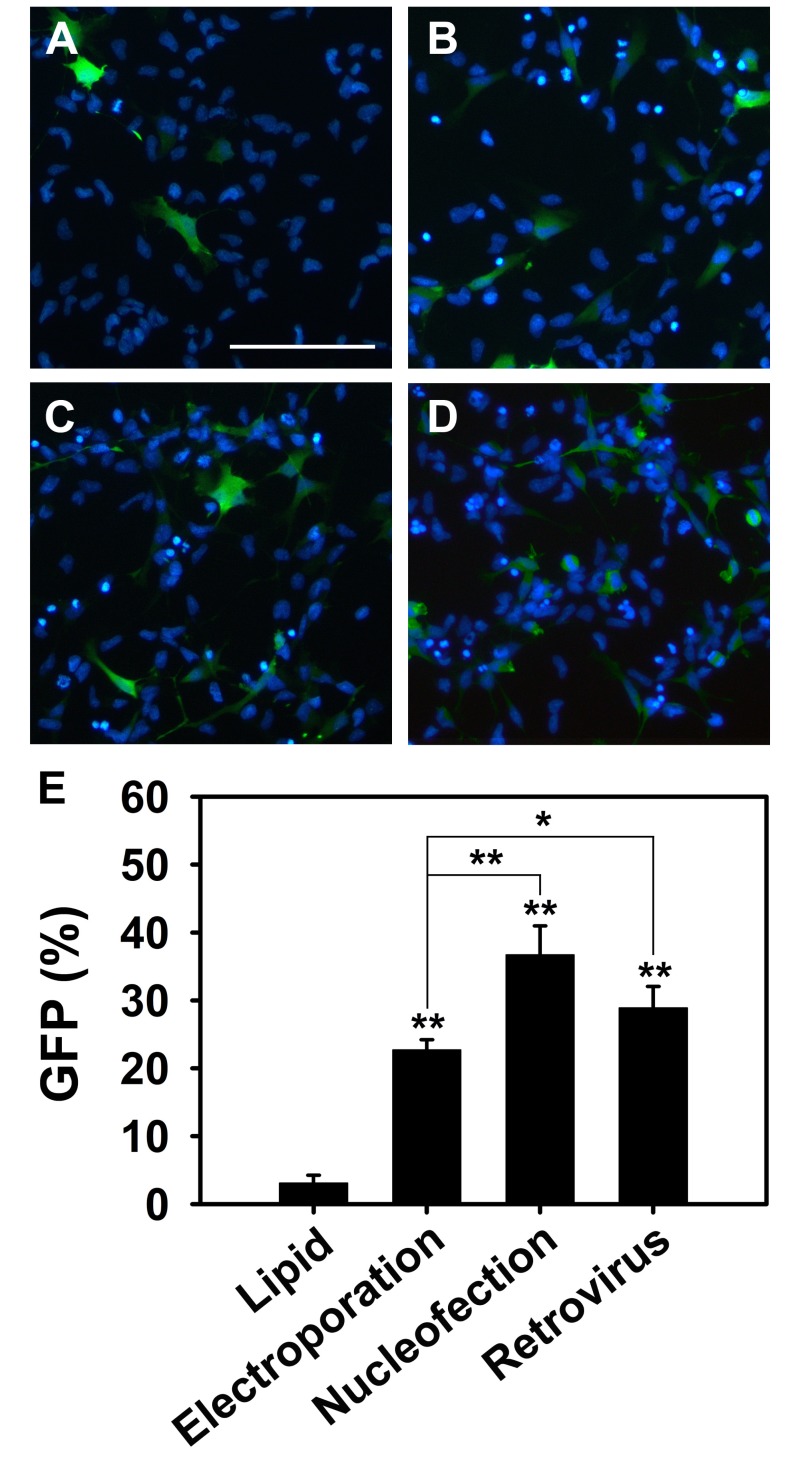
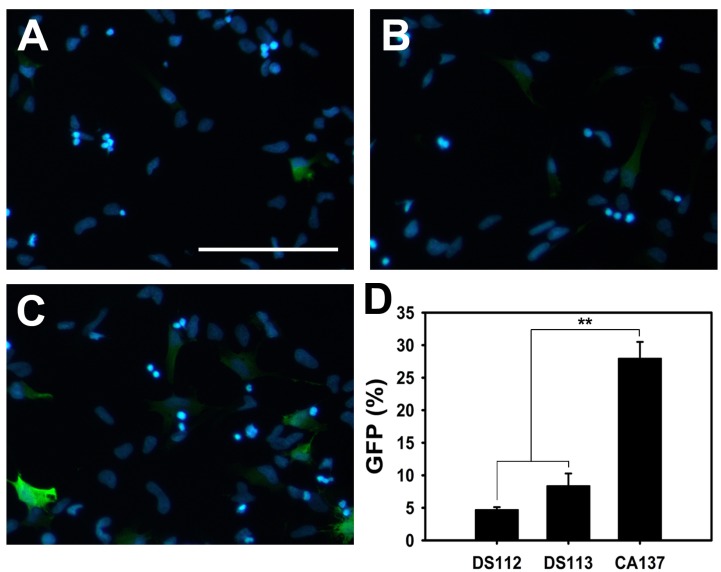
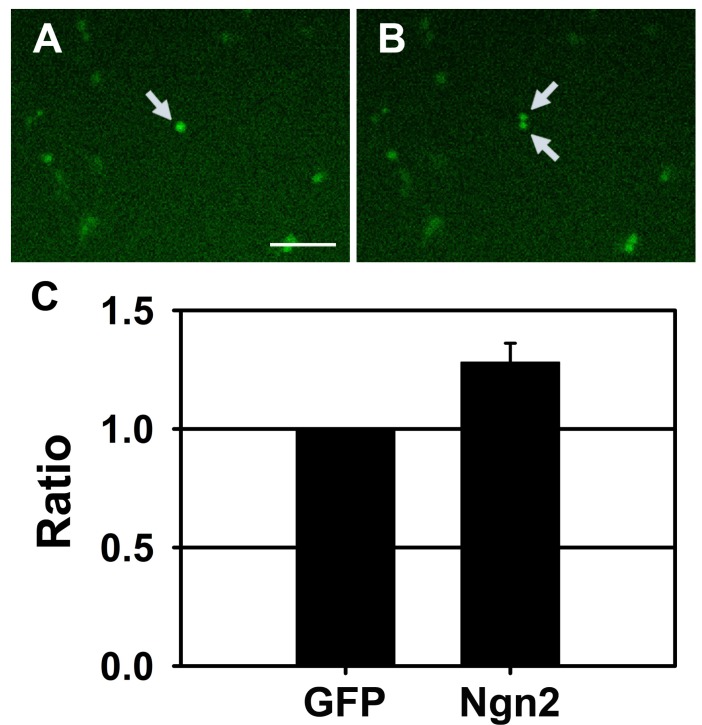
- Neural stem cells (NSCs) have the ability to proliferate and differentiate into various types of cells that compose the nervous system. To study functions of genes in stem cell biology, genes or siRNAs need to be transfected. However, it is difficult to transfect ectopic genes into NSCs. Thus to identify the suitable method to achieve high transfection efficiency, we compared lipid transfection, electroporation, nucleofection and retroviral transduction. Among the methods that we tested, we found that nucleofection and retroviral transduction showed significantly increased transfection efficiency. In addition, with retroviral transduction of Ngn2 that is known to induce neurogenesis in various types of cells, we observed facilitated final cell division in rat NSCs. These data suggest that nucleofection and retroviral transduction provide high efficiency of gene delivery system to study functions of genes in rat NSCs.
MeSH Terms
Figure
Reference
-
1. Capowski EE, Schneider BL, Ebert AD, Seehus CR, Szulc J, Zufferey R, Aebischer P, Svendsen CN. Lentiviral vector-mediated genetic modification of human neural progenitor cells for ex vivo gene therapy. J Neurosci Methods. 2007; 163:338–349. PMID: 17397931.
Article2. Kim HJ. Stem cell potential in Parkinson's disease and molecular factors for the generation of dopamine neurons. Biochim Biophys Acta. 2011; 1812:1–11. PMID: 20713152.
Article3. Müller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006; 7:75–84. PMID: 16371952.
Article4. Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008; 451:937–942. PMID: 18288183.
Article5. Kim HJ, Jin CY. Stem cells in drug screening for neurodegenerative disease. Korean J Physiol Pharmacol. 2012; 16:1–9. PMID: 22416213.
Article6. Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001; 294:2186–2189. PMID: 11691952.7. Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000; 287:1489–1493. PMID: 10688798.
Article8. Van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009; 136:903–912. PMID: 19269367.
Article9. Bauer M, Meyer M, Sautter J, Gasser T, Ueffing M, Widmer HR. Liposome-mediated gene transfer to fetal human ventral mesencephalic explant cultures. Neurosci Lett. 2001; 308:169–172. PMID: 11479015.
Article10. Kim YC, Shim JW, Oh YJ, Son H, Lee YS, Lee SH. Co-transfection with cDNA encoding the Bcl family of anti-apoptotic proteins improves the efficiency of transfection in primary fetal neural stem cells. J Neurosci Methods. 2002; 117:153–158. PMID: 12100980.
Article11. Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, Verfaillie CM. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004; 22:531–543. PMID: 15277699.
Article12. Cesnulevicius K, Timmer M, Wesemann M, Thomas T, Barkhausen T, Grothe C. Nucleofection is the most efficient nonviral transfection method for neuronal stem cells derived from ventral mesencephali with no changes in cell composition or dopaminergic fate. Stem Cells. 2006; 24:2776–2791. PMID: 16902196.
Article13. Tinsley RB, Faijerson J, Eriksson PS. Efficient non-viral transfection of adult neural stem/progenitor cells, without affecting viability, proliferation or differentiation. J Gene Med. 2006; 8:72–81. PMID: 16097040.
Article15. Brüstle O, McKay RD. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996; 6:688–695. PMID: 8937835.
Article16. Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009; 6:e1000029. PMID: 19226183.
Article17. Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007; 20:688–692. PMID: 17992090.
Article18. Bertram B, Wiese S, von Holst A. High-efficiency transfection and survival rates of embryonic and adult mouse neural stem cells achieved by electroporation. J Neurosci Methods. 2012; 209:420–427. PMID: 22750652.
Article19. Osório J, Mueller T, Rétaux S, Vernier P, Wullimann MF. Phylotypic expression of the bHLH genes Neurogenin2, Neurod, and Mash1 in the mouse embryonic forebrain. J Comp Neurol. 2010; 518:851–871. PMID: 20058311.20. Kim HJ, McMillan E, Han F, Svendsen CN. Regionally specified human neural progenitor cells derived from the mesencephalon and forebrain undergo increased neurogenesis following overexpression of ASCL1. Stem Cells. 2009; 27:390–398. PMID: 19008346.
Article21. Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005; 132:2709–2719. PMID: 15901662.
Article22. Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007; 134:1617–1629. PMID: 17344230.
Article23. Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002; 3:517–530. PMID: 12094208.
Article24. Kim HJ, Sugimori M, Nakafuku M, Svendsen CN. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007; 203:394–405. PMID: 17034791.
Article25. Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003; 62:27–34. PMID: 12719940.
Article26. Fraley R, Subramani S, Berg P, Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980; 255:10431–10435. PMID: 6253474.
Article27. Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987; 84:7413–7417. PMID: 2823261.
Article28. Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, Colombo MP, Versura P, D'Errico-Grigioni A, Ferri E, Baccarani M, Lemoli RM. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006; 24:454–461. PMID: 16099993.
Article29. Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001; 240:237–246. PMID: 11784059.30. Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001; 103:865–872. PMID: 11301197.
Article31. Mertz KD, Weisheit G, Schilling K, Lüers GH. Electroporation of primary neural cultures: a simple method for directed gene transfer in vitro. Histochem Cell Biol. 2002; 118:501–506. PMID: 12483315.32. Richard I, Ader M, Sytnyk V, Dityatev A, Richard G, Schachner M, Bartsch U. Electroporation-based gene transfer for efficient transfection of neural precursor cells. Brain Res Mol Brain Res. 2005; 138:182–190. PMID: 15908040.
Article33. De Vry J, Martínez-Martínez P, Losen M, Temel Y, Steckler T, Steinbusch HW, De Baets MH, Prickaerts J. In vivo electroporation of the central nervous system: a non-viral approach for targeted gene delivery. Prog Neurobiol. 2010; 92:227–244. PMID: 20937354.34. Dityateva G, Hammond M, Thiel C, Ruonala MO, Delling M, Siebenkotten G, Nix M, Dityatev A. Rapid and efficient electroporation-based gene transfer into primary dissociated neurons. J Neurosci Methods. 2003; 130:65–73. PMID: 14583405.
Article35. Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK. Efficient transfection method for primary cells. Tissue Eng. 2002; 8:235–245. PMID: 12031113.
Article36. Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES, Körner I, Gan L, Chen S, Castro-Obregon S, Hammermann R, Wolf J, Müller-Hartmann H, Nix M, Siebenkotten G, Kraus G, Lun K. New non-viral method for gene transfer into primary cells. Methods. 2004; 33:151–163. PMID: 15121170.
Article37. Siemen H, Nix M, Endl E, Koch P, Itskovitz-Eldor J, Brüstle O. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005; 14:378–383. PMID: 16137226.
Article38. Von Levetzow G, Spanholtz J, Beckmann J, Fischer J, Kögler G, Wernet P, Punzel M, Giebel B. Nucleofection, an efficient nonviral method to transfer genes into human hematopoietic stem and progenitor cells. Stem Cells Dev. 2006; 15:278–285. PMID: 16646674.39. Dieterlen MT, Wegner F, Schwarz SC, Milosevic J, Schneider B, Busch M, Römuss U, Brandt A, Storch A, Schwarz J. Non-viral gene transfer by nucleofection allows stable gene expression in human neural progenitor cells. J Neurosci Methods. 2009; 178:15–23. PMID: 19059435.
Article40. Cone RD, Mulligan RC. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci USA. 1984; 81:6349–6353. PMID: 6093098.
Article41. Eglitis MA, Kantoff P, Gilboa E, Anderson WF. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985; 230:1395–1398. PMID: 2999985.
Article42. Park SW, Won KJ, Lee YS, Kim HS, Kim YK, Lee HW, Kim B, Lee BH, Kim JH, Kim DK. Increased HoxB4 inhibits apoptotic cell death in Pro-B cells. Korean J Physiol Pharmacol. 2012; 16:265–271. PMID: 22915992.
Article43. Park KS, Wiederkehr A, Wollheim CB. Defective mitochondrial function and motility due to mitofusin 1 overexpression in insulin secreting cells. Korean J Physiol Pharmacol. 2012; 16:71–77. PMID: 22416223.
Article44. Lu H, Li M, Song T, Qian Y, Xiao X, Chen X, Zhang P, Feng X, Parker T, Liu Y. Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res Bull. 2008; 77:158–164. PMID: 19875351.45. Ostenfeld T, Tai YT, Martin P, Déglon N, Aebischer P, Svendsen CN. Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons. J Neurosci Res. 2002; 69:955–965. PMID: 12205689.
Article46. Kameda M, Shingo T, Takahashi K, Muraoka K, Kurozumi K, Yasuhara T, Maruo T, Tsuboi T, Uozumi T, Matsui T, Miyoshi Y, Hamada H, Date I. Adult neural stem and progenitor cells modified to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur J Neurosci. 2007; 26:1462–1478. PMID: 17880388.
Article47. Hirsch ML, Fagan BM, Dumitru R, Bower JJ, Yadav S, Porteus MH, Pevny LH, Samulski RJ. Viral single-strand DNA induces p53-dependent apoptosis in human embryonic stem cells. PLoS One. 2011; 6:e27520. PMID: 22114676.
Article48. Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990; 10:4239–4242. PMID: 2370865.
Article49. Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993; 12:2099–2108. PMID: 8491198.
Article50. Kulkosky J, Skalka AM. Molecular mechanism of retroviral DNA integration. Pharmacol Ther. 1994; 61:185–203. PMID: 7938170.
Article51. Goff SP. Genetics of retroviral integration. Annu Rev Genet. 1992; 26:527–544. PMID: 1482125.
Article52. Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998; 9:457–463. PMID: 9821272.
Article53. Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992; 11:3053–3058. PMID: 1322294.
Article54. Luskey BD, Rosenblatt M, Zsebo K, Williams DA. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells. Blood. 1992; 80:396–402. PMID: 1378319.55. Elwood NJ, Zogos H, Willson T, Begley CG. Retroviral transduction of human progenitor cells: use of granulocyte colony-stimulating factor plus stem cell factor to mobilize progenitor cells in vivo and stimulation by Flt3/Flk-2 ligand in vitro. Blood. 1996; 88:4452–4462. PMID: 8977237.56. Orlic D, Girard LJ, Anderson SM, Barrette S, Broxmeyer HE, Bodine DM. Amphotropic retrovirus transduction of hematopoietic stem cells. Ann N Y Acad Sci. 1999; 872:115–123. PMID: 10372116.
Article57. Rothe M, Rittelmeyer I, Iken M, Rüdrich U, Schambach A, Glage S, Manns MP, Baum C, Bock M, Ott M, Modlich U. Epidermal growth factor improves lentivirus vector gene transfer into primary mouse hepatocytes. Gene Ther. 2012; 19:425–434. PMID: 21850050.
Article58. Kim E, Oh JS, Ahn IS, Park KI, Jang JH. Magnetically enhanced adeno-associated viral vector delivery for human neural stem cell infection. Biomaterials. 2011; 32:8654–8662. PMID: 21840595.
Article59. Kim JS, Chu HS, Park KI, Won JI, Jang JH. Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene Ther. 2012; 19:329–337. PMID: 21654823.
Article60. Lee YH, Peng CA. Enhanced retroviral gene delivery in ultrasonic standing wave fields. Gene Ther. 2005; 12:625–633. PMID: 15647763.
Article61. Naka T, Sakoda T, Doi T, Tsujino T, Masuyama T, Kawashima S, Iwasaki T, Ohyanagi M. Ultrasound enhances retrovirus-mediated gene transfer. Prep Biochem Biotechnol. 2007; 37:87–99. PMID: 17454820.
Article62. Porter CD, Lukacs KV, Box G, Takeuchi Y, Collins MK. Cationic liposomes enhance the rate of transduction by a recombinant retroviral vector in vitro and in vivo. J Virol. 1998; 72:4832–4840. PMID: 9573249.63. Themis M, Forbes SJ, Chan L, Cooper RG, Etheridge CJ, Miller AD, Hodgson HJ, Coutelle C. Enhanced in vitro and in vivo gene delivery using cationic agent complexed retrovirus vectors. Gene Ther. 1998; 5:1180–1186. PMID: 9930318.64. Coelen RJ, Jose DG, May JT. The effect of hexadimethrine bromide (polybrene) on the infection of the primate retroviruses SSV 1/SSAV 1 and BaEV. Arch Virol. 1983; 75:307–311. PMID: 6301409.
Article65. Coller BS. Polybrene-induced platelet agglutination and reduction in electrophoretic mobility: enhancement by von Willebrand factor and inhibition by vancomycin. Blood. 1980; 55:276–281. PMID: 6766335.
Article66. Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys J. 2004; 86:1234–1242. PMID: 14747357.
Article67. Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988; 32:1742–1745. PMID: 2472775.
Article68. Batra RK, Olsen JC, Hoganson DK, Caterson B, Boucher RC. Retroviral gene transfer is inhibited by chondroitin sulfate proteoglycans/glycosaminoglycans in malignant pleural effusions. J Biol Chem. 1997; 272:11736–11743. PMID: 9115227.
Article69. Toyoshima K, Vogt PK. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969; 38:414–426. PMID: 4308055.
Article70. Le Doux JM, Landazuri N, Yarmush ML, Morgan JR. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum Gene Ther. 2001; 12:1611–1621. PMID: 11535165.
Article71. Kaneko Y, Tsukamoto A. Structural characteristics of cationic liposomes with potent enhancing effect on retroviral transduction into human hepatoma cells. Cancer Lett. 1996; 107:211–215. PMID: 8947515.
Article72. Hodgson CP, Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol. 1996; 14:339–342. PMID: 9630897.
Article73. Jang J, Lee J, Kim ST, Lee KY, Cho JY, Kweon DH, Kwon ST, Koh YH, Kim S, Yoon K. Polycation-mediated enhancement of retroviral transduction efficiency depends on target cell types and pseudotyped Env proteins: implication for gene transfer into neural stem cells. Neurochem Int. 2012; 60:846–851. PMID: 22421532.
Article74. Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000; 127:693–702. PMID: 10648228.
Article75. Thoma EC, Wischmeyer E, Offen N, Maurus K, Sirén AL, Schartl M, Wagner TU. Ectopic expression of neurogenin 2 alone is sufficient to induce differentiation of embryonic stem cells into mature neurons. PLoS One. 2012; 7:e38651. PMID: 22719915.
Article76. Ribes V, Stutzmann F, Bianchetti L, Guillemot F, Dollé P, Le Roux I. Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev Biol. 2008; 321:470–481. PMID: 18590718.
Article77. Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005; 17:648–657. PMID: 16243506.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and Characterization of Chicken NPAS3
- Neural Stem Cells
- The Effect of Recombinant Tyrosine Hydroxylase Expression on the Neurogenic Differentiation Potency of Mesenchymal Stem Cells
- Transplantation of neural stem cells: cellular & gene therapy for hypoxic-ischemic brain injury
- Differentiation of Rat Neural Stem Cells Following Transplantation in the Brain of Huntington's Disease Rat Model