Cancer Res Treat.
2023 Apr;55(2):626-635. 10.4143/crt.2022.1058.
Trastuzumab Combined with Irinotecan in Patients with HER2-Positive Metastatic Colorectal Cancer: A Phase II Single-Arm Study and Exploratory Biomarker Analysis
- Affiliations
-
- 1Department of Gastrointestinal Oncology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, China
- 2The Medical Department, 3D Medicines Inc., Shanghai, China
- 3Department of Early Drug Development Center, Peking University Cancer Hospital and Institute, Beijing, china
- 4Department of Pathology, Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education, Peking University Cancer Hospital and Institute, Beijing, China
- KMID: 2541249
- DOI: http://doi.org/10.4143/crt.2022.1058
Abstract
- Purpose
The human epidermal growth factor receptor 2 (HER2) is an established therapeutic target for various kinds of solid tumors. HER2 amplification occurs in approximately 1% to 6% of colorectal cancer. In this study, we aimed to assess the efficacy and safety of trastuzumab in combination with chemotherapy in HER2-positive metastatic colorectal cancer (mCRC).
Materials and Methods
An open-label, phase II trial (Clinicaltrials.gov: NCT03185988) was designed to evaluate the antitumor activity of trastuzumab and chemotherapy in HER2-positive digestive cancers excluding gastric cancer in 2017. Patients from this trial with HER2-positive, KRAS/BRAF wild-type, unresectable mCRC were analyzed in this manuscript. Eligible patients were treated with trastuzumab (8 mg/kg loading dose and then 6 mg/kg every 3 weeks) and irinotecan (120 mg/m2 days 1 and 8 every 3 weeks). The primary endpoint was the objective response rate.
Results
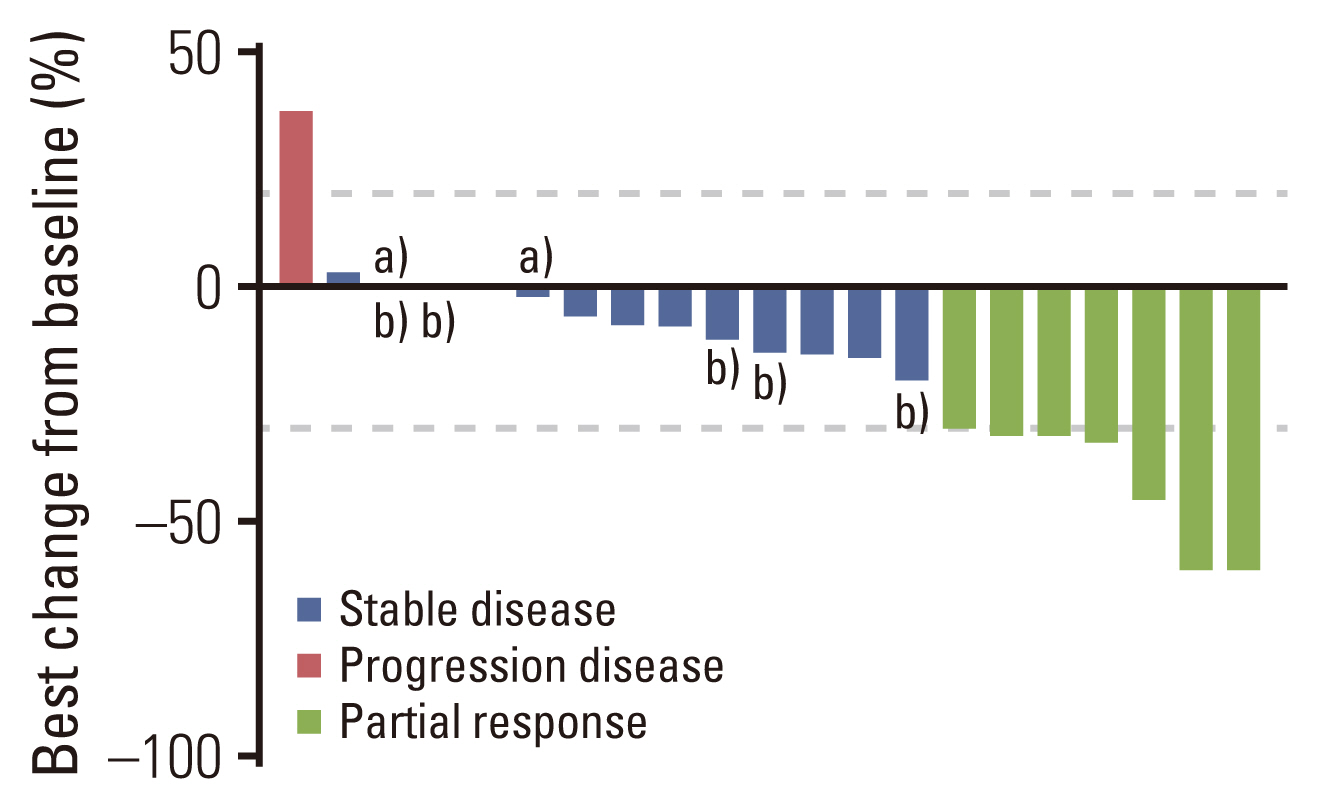
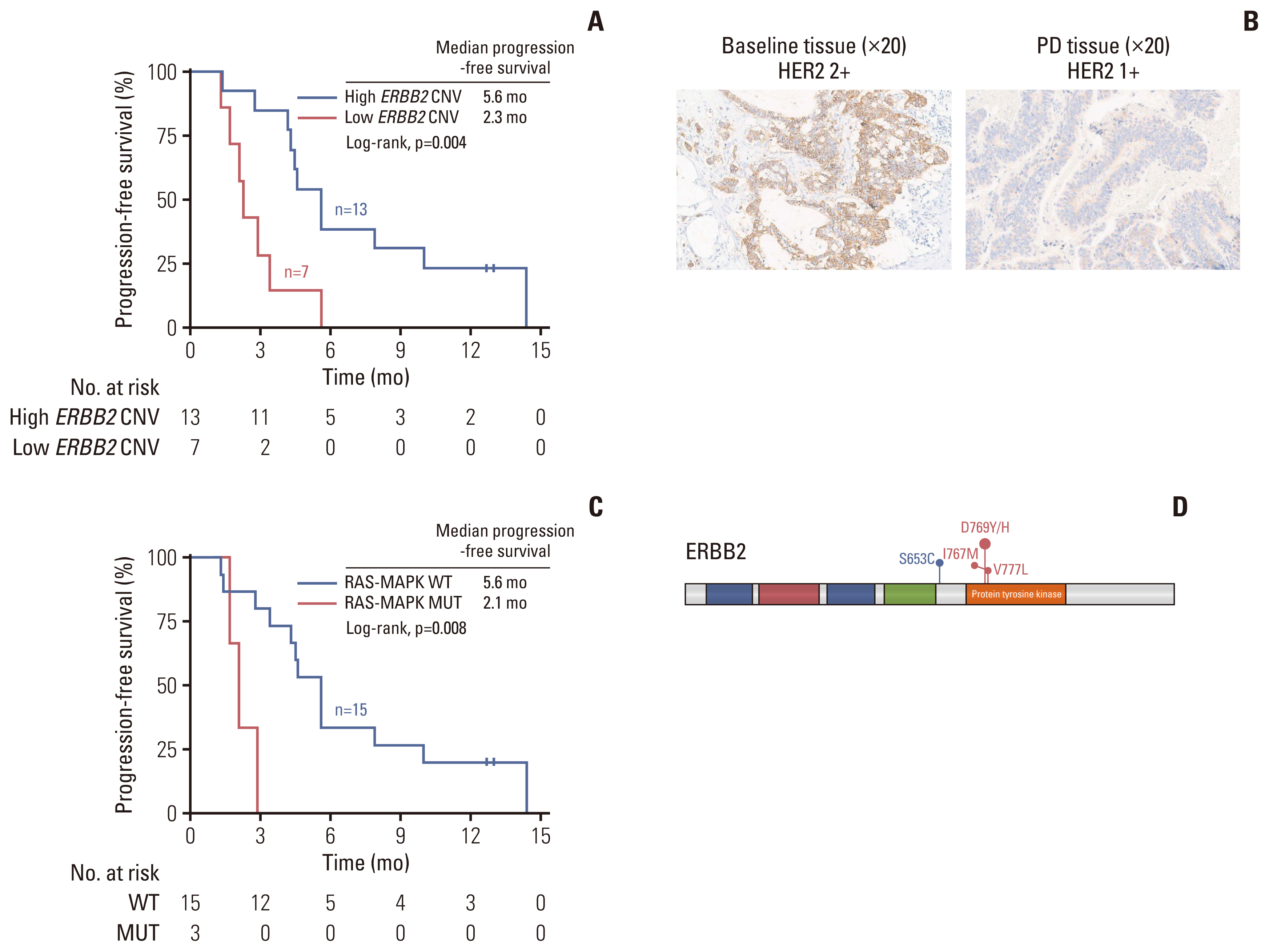
Twenty-one HER2-positive mCRC patients were enrolled in this study. Seven patients (33.3%) achieved an objective res-ponse, and 11 patients (52.4%) had stable disease as their best response. The median progression-free survival (PFS) was 4.3 months (95% confidence interval, 2.7 to 5.9). Four of the 21 patients (19.0%) had grade 3 adverse events, including leukopenia, neutropenia, urinary tract infection, and diarrhea. No treatment-related death was reported. Exploratory analyses revealed that high tumor tissue HER2 copy number was associated with better therapeutic response and PFS. Alterations in the mitogen-activated protein kinase pathway, HER2 gene, phosphoinositide 3-kinase/AKT pathway, and cell cycle control genes were potential drivers of trastuzumab resistance in mCRC.
Conclusion
Trastuzumab combined with chemotherapy is a potentially effective and well-tolerated therapeutic regimen in mCRC with a high HER2 copy number.
Figure
Reference
-
References
1. Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018; 29:1108–19.
Article2. Whenham N, D’Hondt V, Piccart MJ. HER2-positive breast cancer: from trastuzumab to innovatory anti-HER2 strategies. Clin Breast Cancer. 2008; 8:38–49.
Article3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article4. Kreutzfeldt J, Rozeboom B, Dey N, De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020; 10:1045–67.5. Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020; 17:33–48.
Article6. Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, et al. Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol. 2013; 37:522–31.
Article7. Wei Q, Zhang Y, Gao J, Li J, Li J, Li Y, et al. Clinicopathologic characteristics of HER2-positive metastatic colorectal cancer and detection of HER2 in plasma circulating tumor DNA. Clin Colorectal Cancer. 2019; 18:175–82.
Article8. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012; 487:330–7.9. Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015; 28:1481–91.
Article10. Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013; 108:668–75.
Article11. Bregni G, Sciallero S, Sobrero A. HER2 amplification and anti-EGFR sensitivity in advanced colorectal cancer. JAMA Oncol. 2019; 5:605–6.
Article12. Clark JW, Niedzwiecki D, Hollis D, Mayer R. Phase-II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzamab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. Onkologie. 2003; 26(Suppl 3):13–46.13. Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy: a phase II trial. Cancer Invest. 2004; 22:858–65.
Article14. Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012; 109:14476–81.15. Nagano M, Kohsaka S, Ueno T, Kojima S, Saka K, Iwase H, et al. High-throughput functional evaluation of variants of unknown significance in ERBB2. Clin Cancer Res. 2018; 24:5112–22.16. Mitra D, Brumlik MJ, Okamgba SU, Zhu Y, Duplessis TT, Parvani JG, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther. 2009; 8:2152–62.
Article17. Castagnoli L, Iezzi M, Ghedini GC, Ciravolo V, Marzano G, Lamolinara A, et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014; 74:6248–59.
Article18. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010; 28:4706–13.
Article19. Xu RH, Muro K, Morita S, Iwasa S, Han SW, Wang W, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, openlabel, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018; 19:660–71.
Article20. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:303–12.
Article21. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018; 319:2486–96.
Article22. Meric-Bernstam F, Hurwitz H, Raghav KP, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019; 20:518–30.
Article23. Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016; 17:738–46.
Article24. Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int J Cancer. 2016; 139:2859–64.
Article25. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018; 36:536–42.
Article26. Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell. 2018; 34:148–62.
Article27. Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012; 18:6784–91.
Article28. Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019; 68:1152–61.
Article29. Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011; 108:3761–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trastuzumab Biosimilar (HLX02), Pertuzumab Plus Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer after Progression of Trastuzumab: A Prospective, Phase II Study
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Nine months versus 12 months of adjuvant trastuzumab for patients with HER2-positive breast cancer
- Pyrotinib Combined with Vinorelbine in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer: A Multicenter, Single-Arm, Prospective Study
- Comparison of survival outcomes according of patients with metastatic gastric cancer receiving trastuzumab with systemic chemotherapy