Cancer Res Treat.
2024 Apr;56(2):513-521. 10.4143/crt.2023.786.
Pyrotinib Combined with Vinorelbine in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer: A Multicenter, Single-Arm, Prospective Study
- Affiliations
-
- 1Department of Medical Oncology, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2Department of Radiology, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Department of Medical Oncology, Meizhou People's Hospital (Huangtang Hospital), Meizhou, China
- 4Department of Oncology Surgery, Shantou Central Hospital, Shantou, China
- KMID: 2554340
- DOI: http://doi.org/10.4143/crt.2023.786
Abstract
- Purpose
This study aims to evaluate the efficacy and safety of a new combination treatment of vinorelbine and pyrotinib in human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer (MBC) and provide higher level evidence for clinical practice.
Materials and Methods
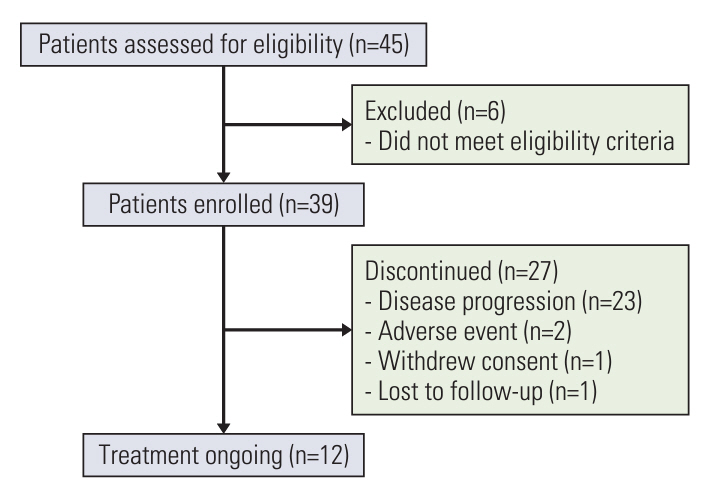
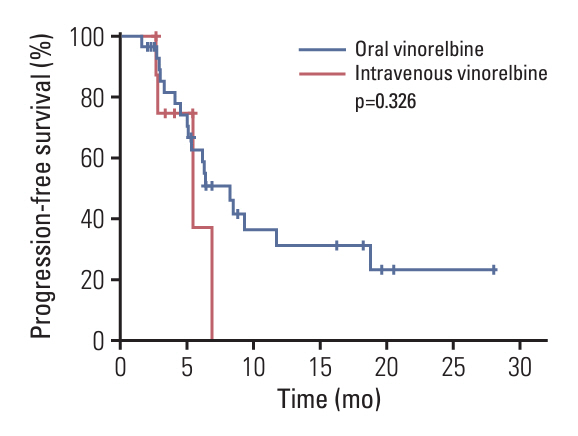
This was a prospective, single-arm, phase 2 trial conducted at three institutions in China. Patients with HER2-positive MBC, who had previously been treated with trastuzumab plus a taxane or trastuzumab plus pertuzumab combined with a chemotherapeutic agent, were enrolled between March 2020 and December 2021. All patients received pyrotinib 400 mg orally once daily plus vinorelbine 25 mg/m2 intravenously or 60-80 mg/m2 orally on day 1 and day 8 of 21-day cycle. The primary endpoint was progression-free survival (PFS), and the secondary endpoints included the objective response rate (ORR), disease control rate (DCR), overall survival, and safety.
Results
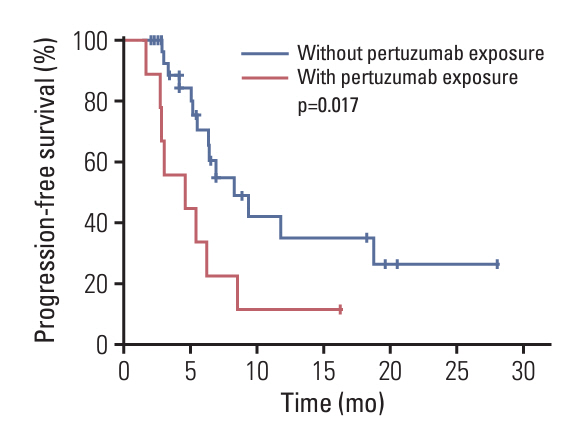
A total of 39 patients were enrolled. All patients had been pretreated with trastuzumab and 23.1% (n=9) of them had accepted trastuzumab plus pertuzumab. The median follow-up time was 16.3 months (95% confidence interval [CI], 5.3 to 27.2), and the median PFS was 6.4 months (95% CI, 4.0 to 8.8). The ORR was 43.6% (95% CI, 27.8% to 60.4%) and the DCR was 84.6% (95% CI, 69.5% to 94.1%). The median PFS of patients with versus without prior pertuzumab treatment was 4.6 and 8.3 months (p=0.017). The most common grade 3/4 adverse events were diarrhea (28.2%), neutrophil count decreased (15.4%), white blood cell count decreased (7.7%), vomiting (5.1%), and anemia (2.6%).
Conclusion
Pyrotinib plus vinorelbine showed promising efficacy and tolerable toxicity as second-line treatment in patients with HER2-positive MBC.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019; 321:288–300.3. Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004; 5:63–9.
Article4. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.
Article5. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015; 372:724–34.
Article6. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367:1783–91.
Article7. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022; 386:1143–54.
Article8. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006; 355:2733–43.
Article9. Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016; 2:1557–64.
Article10. Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017; 110:51–61.
Article11. Gourd E. Pyrotinib shows activity in metastatic breast cancer. Lancet Oncol. 2017; 18:e643.
Article12. Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019; 25:5212–20.
Article13. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019; 37:2610–9.
Article14. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021; 22:351–60.
Article15. Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004; 96:739–49.
Article16. Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007; 110:965–72.
Article17. Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011; 29:264–71.
Article18. Li Y, Qiu Y, Li H, Luo T, Li W, Wang H, et al. Pyrotinib combined with vinorelbine in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol. 2021; 11:664429.
Article19. Xie Y, Li Y, Ting L, Sang D, Yuan P, Li W, et al. Pyrotinib plus vinorelbine versus lapatinib plus capecitabine in patients with previously treated HER2-positive metastatic breast cancer: a multicenter, retrospective study. Front Oncol. 2021; 11:699333.
Article20. Yin S, Chi Y, Du Y, Wang J, Shan C, Yi W, et al. Efficacy and safety of pyrotinib-containing regimen in the patients with HER2-positive metastatic breast cancer: a multicenter real-world study. Cancer Med. 2023; 12:2333–44.
Article21. Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018; 36:2105–22.
Article22. Yan M, Bian L, Hu X, Zhang Q, Ouyang Q, Feng J, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res. 2020; 1:1–13.
Article23. Singla H, Kalra S, Kheterpal P, Kumar V, Munshi A. Role of genomic alterations in HER2 positive breast carcinoma: focus on susceptibility and trastuzumab-therapy. Curr Cancer Drug Targets. 2017; 17:344–56.
Article24. Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012; 2:62.
Article25. Singla H, Munshi A. HER2 tyrosine kinase inhibitors in the sensitization to cancers resistant to HER2 antibodies. Crit Rev Oncog. 2020; 25:241–50.
Article26. Su B, Huang T, Jin Y, Yin H, Qiu H, Yuan X. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021; 24:352–67.
Article27. Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, et al. Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol. 2001; 12:1643–9.
Article28. Bonneterre J, Chevalier B, Focan C, Mauriac L, Piccart M. Phase I and pharmacokinetic study of weekly oral therapy with vinorelbine in patients with advanced breast cancer (ABC). Ann Oncol. 2001; 12:1683–91.
Article29. Petain A, Zhong D, Chen X, Li Z, Zhimin S, Zefei J, et al. Effect of ethnicity on vinorelbine pharmacokinetics: a population pharmacokinetics analysis. Cancer Chemother Pharmacol. 2019; 84:373–82.
Article30. Jassem J, Ramlau R, Karnicka-Mlodkowska H, Krawczyk K, Krzakowski M, Zatloukal P, et al. A multicenter randomized phase II study of oral vs. intravenous vinorelbine in advanced non-small-cell lung cancer patients. Ann Oncol. 2001; 12:1375–81.
Article31. Yang Y, Chang J, Huang C, Zhang Y, Wang J, Shu Y, et al. A randomised, multicentre open-label phase II study to evaluate the efficacy, tolerability and pharmacokinetics of oral vinorelbine plus cisplatin versus intravenous vinorelbine plus cisplatin in Chinese patients with chemotherapy-naive unresectable or metastatic non-small cell lung cancer. J Thorac Dis. 2019; 11:3347–59.
Article32. Huang L, Wang X, Zhou L, Di L, Zheng H, Jiang Z, et al. Oral vinorelbine versus intravenous vinorelbine, in combination with epirubicin as first-line chemotherapy in Chinese patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2020; 85:205–15.
Article33. Schott S, Schneeweiss A, Reinhardt J, Bruckner T, Domschke C, Sohn C, et al. Acceptance of oral chemotherapy in breast cancer patients: a survey study. BMC Cancer. 2011; 11:129.34. Strada MR, Palumbo R, Bernardo A, Riccardi A, Teragni C, Poggi G, et al. Intravenous or oral vinorelbine plus capecitabine as first-line treatment in HER2- metastatic breast cancer: joint analysis of 2 consecutive prospective phase II trials. Clin Breast Cancer. 2012; 12:30–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis
- Trastuzumab Biosimilar (HLX02), Pertuzumab Plus Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer after Progression of Trastuzumab: A Prospective, Phase II Study
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Phase II Study of Vinorelbine Plus Trastuzumab in HER2 Overexpressing Metastatic Breast Cancer Pretreated with Anthracyclines and Taxanes
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer