Cancer Res Treat.
2024 Jul;56(3):795-801. 10.4143/crt.2023.1151.
Trastuzumab Biosimilar (HLX02), Pertuzumab Plus Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer after Progression of Trastuzumab: A Prospective, Phase II Study
- Affiliations
-
- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Breast Oncology, Peking University Cancer Hospital and Institute, Beijing, China
- 2State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing, China
- KMID: 2557666
- DOI: http://doi.org/10.4143/crt.2023.1151
Abstract
- Purpose
This study aims to evaluate the efficacy and safety of trastuzumab biosimilar (HLX02) in combination with pertuzumab and chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer (MBC) after progression of trastuzumab.
Materials and Methods
In this prospective, single-arm, phase II study, patients with HER2-positive MBC after progression of trastuzumab received pertuzuamb, HLX02, and chemotherapy in Beijing Cancer Hospital from March 2020 to December 2022. The primary endpoint was progression-free survival (PFS), and secondary endpoints included objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety. The study was registered with ClinicalTrials.gov (NCT05188495).
Results
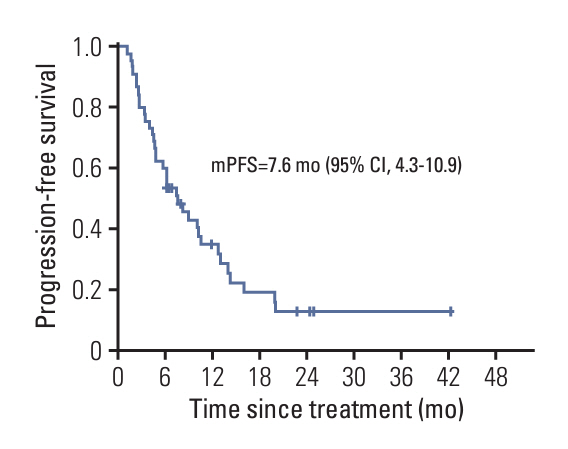
A total of 45 patients were included in this study. Twelve patients (26.7%) were treated in second-line and 33 patients (73.3%) were in third-line and later setting. Eighty percent and 15.5% patients had previously received pyrotinib/lapatinib and T-DM1, respectively. With a median follow-up of 24.4 months (range, 1.2 to 43.9 months), the median PFS was 7.6 months (95% confidence interval, 4.3 to 10.9), OS was not reached, the ORR was 31.1%, and DCR was 91.1%. The treatment was well tolerated.
Conclusion
The combination of trastuzumab biosimilar HLX02, pertuzumab, and chemotherapy exhibited promising efficacy and a favorable safety profile as second- and beyond-line treatment in HER2-positive MBC.
Figure
Reference
-
References
1. Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013; 14:461–71.2. Xu B, Li W, Zhang Q, Li Q, Wang X, Li H, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): final analysis of a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2023; 197:503–13.
Article3. Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023; 22:101–26.
Article4. Tapia M, Hernando C, Martinez MT, Burgues O, TebarSanchez C, Lameirinhas A, et al. Clinical impact of new treatment strategies for HER2-positive metastatic breast cancer patients with resistance to classical anti-HER therapies. Cancers (Basel). 2023; 15:4522.
Article5. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020; 31:1623–49.6. Extra JM, Antoine EC, Vincent-Salomon A, Delozier T, Kerbrat P, Bethune-Volters A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist. 2010; 15:799–809.7. von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011; 47:2273–81.
Article8. Iyengar NM, Smyth LM, Lake D, Gucalp A, Singh JC, Traina TA, et al. Efficacy and safety of gemcitabine with trastuzumab and pertuzumab after prior pertuzumab-based therapy among patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: a phase 2 clinical trial. JAMA Netw Open. 2019; 2:e1916211.9. Dang C, Iyengar N, Datko F, D’Andrea G, Theodoulou M, Dickler M, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015; 33:442–7.
Article10. Yamamoto Y, Iwata H, Taira N, Masuda N, Takahashi M, Yoshinami T, et al. Pertuzumab retreatment for HER2-positive advanced breast cancer: a randomized, open-label phase III study (PRECIOUS). Cancer Sci. 2022; 113:3169–79.
Article11. Yamamoto Y, Iwata H, Ueno T, Taira N, Kashiwaba M, Takahashi M, et al. A randomized, open-label, Phase III trial of pertuzumab retreatment in HER2-positive locally advanced/metastatic breast cancer patients previously treated with pertuzumab, trastuzumab and chemotherapy: the Japan Breast Cancer Research Group-M05 PRECIOUS study. Jpn J Clin Oncol. 2018; 48:855–9.12. Urruticoechea A, Rizwanullah M, Im SA, Sanchez Ruiz AC, Lang I, Tomasello G, et al. Final overall survival (OS) analysis of PHEREXA: a randomized phase III trial of trastuzumab (H) plus capecitabine (X) +/- pertuzumab (P) in patients with HER2-positive metastatic breast cancer (MBC) who experienced disease progression during or after H-based therapy. J Clin Oncol. 2018; 36(15 Suppl):1013.
Article13. Urruticoechea A, Rizwanullah M, Im SA, Sanchez-Ruiz AC, Lang I, Tomasello G, et al. PHEREXA: a phase III study of trastuzumab (H) + capecitabine (X) ± pertuzumab (P) for patients (pts) who progressed during/after one line of H-based therapy in the HER2-positive metastatic breast cancer (MBC) setting. J Clin Oncol. 2016; 34(15 Suppl):504.
Article14. Qian Y, Peng Y, Zhou H, Zhang L, Yuan Y. Trastuzumab plus pertuzumab in combination with chemotherapy in metastatic HER2-positive breast cancer: a retrospective single-armed cohort study in China. Ann Transl Med. 2022; 10:877.
Article15. Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15:689–99.
Article16. Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Musolino A, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021; 7:573–84.17. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): randomised, double blind, multicentre, phase 3 trial. BMJ. 2023; 383:2665.18. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022; 386:1143–54.
Article19. Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017; 18:732–42.
Article20. Welslau M, Dieras V, Sohn JH, Hurvitz SA, Lalla D, Fang L, et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014; 120:642–51.
Article21. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021; 22:351–60.
Article22. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006; 355:2733–43.
Article23. Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020; 38:3138–49.24. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020; 382:610–21.
Article25. Iihara H, Shimokawa M, Bando H, Niwa Y, Mizuno Y, Kawaguchi Y, et al. Doublet or triplet antiemetic prophylaxis for nausea and vomiting induced by trastuzumab deruxtecan: an open-label, randomized, and multicenter exploratory phase 2 Study. J Cancer. 2023; 14:2644–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-World Evidence of Trastuzumab, Pertuzumab, and Docetaxel Combination as a First-Line Treatment for Korean Patients with HER2-Positive Metastatic Breast Cancer
- Nine months versus 12 months of adjuvant trastuzumab for patients with HER2-positive breast cancer
- Intrathecal Trastuzumab Treatment in Patients with Breast Cancer and Leptomeningeal Carcinomatosis
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- The Prediction of HER2-Targeted Treatment Response Using 64CuTetra- Azacyclododecanetetra-Acetic Acid (DOTA)-Trastuzumab PET/CT in Metastatic Breast Cancer: A Case Report