Cancer Res Treat.
2022 Oct;54(4):1130-1137. 10.4143/crt.2021.1103.
Real-World Evidence of Trastuzumab, Pertuzumab, and Docetaxel Combination as a First-Line Treatment for Korean Patients with HER2-Positive Metastatic Breast Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2534192
- DOI: http://doi.org/10.4143/crt.2021.1103
Abstract
- Purpose
Trastuzumab has markedly improved the survival outcomes of patients with human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and dual blockade of HER2 using trastuzumab and pertuzumab in combination with taxanes (THP) has become a standard of care for HER2-positive metastatic breast cancer (MBC) worldwide since the CLEOPATRA trial. We assessed the outcomes of THP as a first-line treatment for Korean HER2-positive MBC patients in the real-world setting.
Materials and Methods
Between August 2008 and October 2020, we identified 228 HER2-positive MBC patients who received THP as a first-line palliative chemotherapy. We analyzed survival outcomes, efficacy, and adverse events of THP retrospectively.
Results
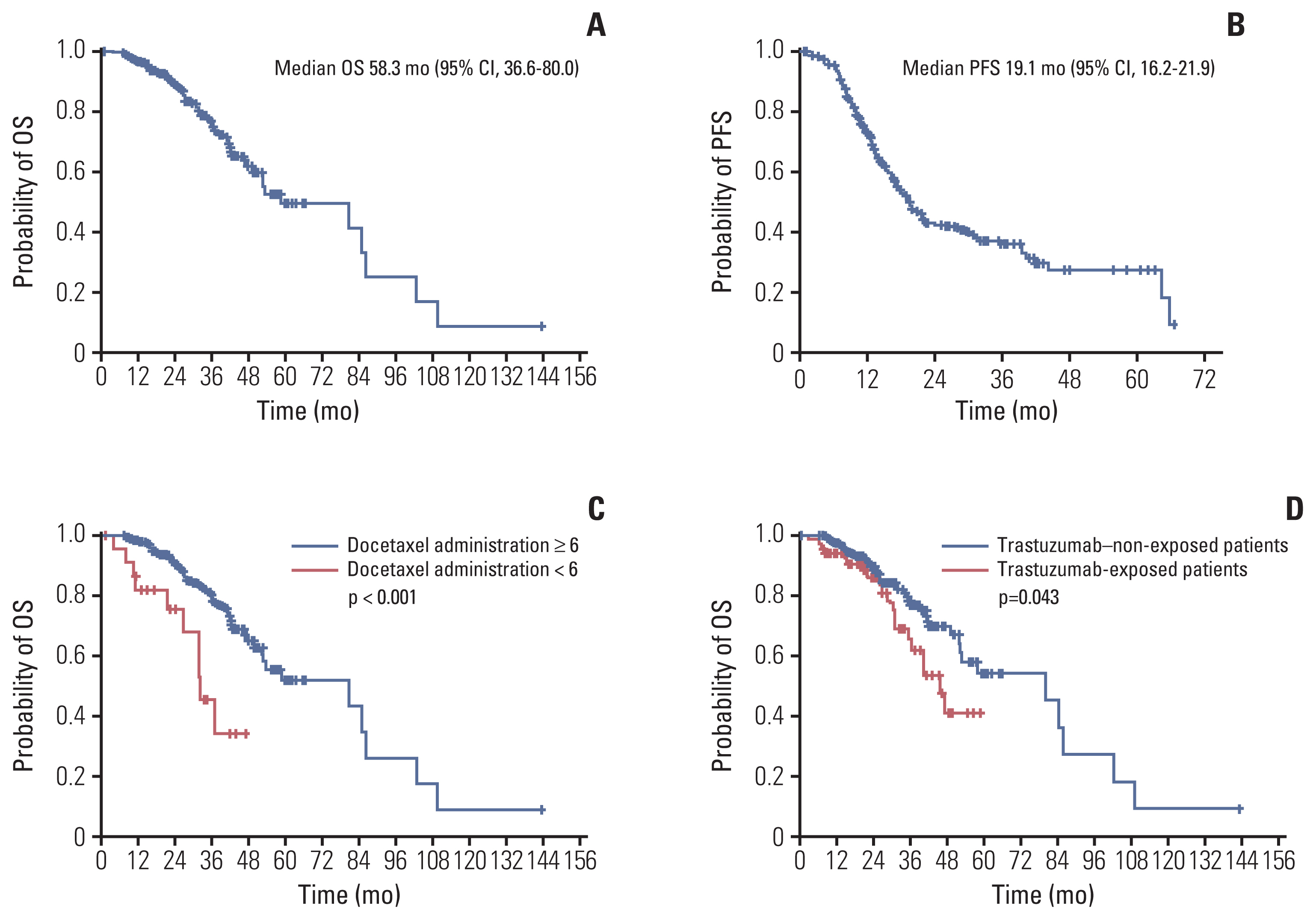
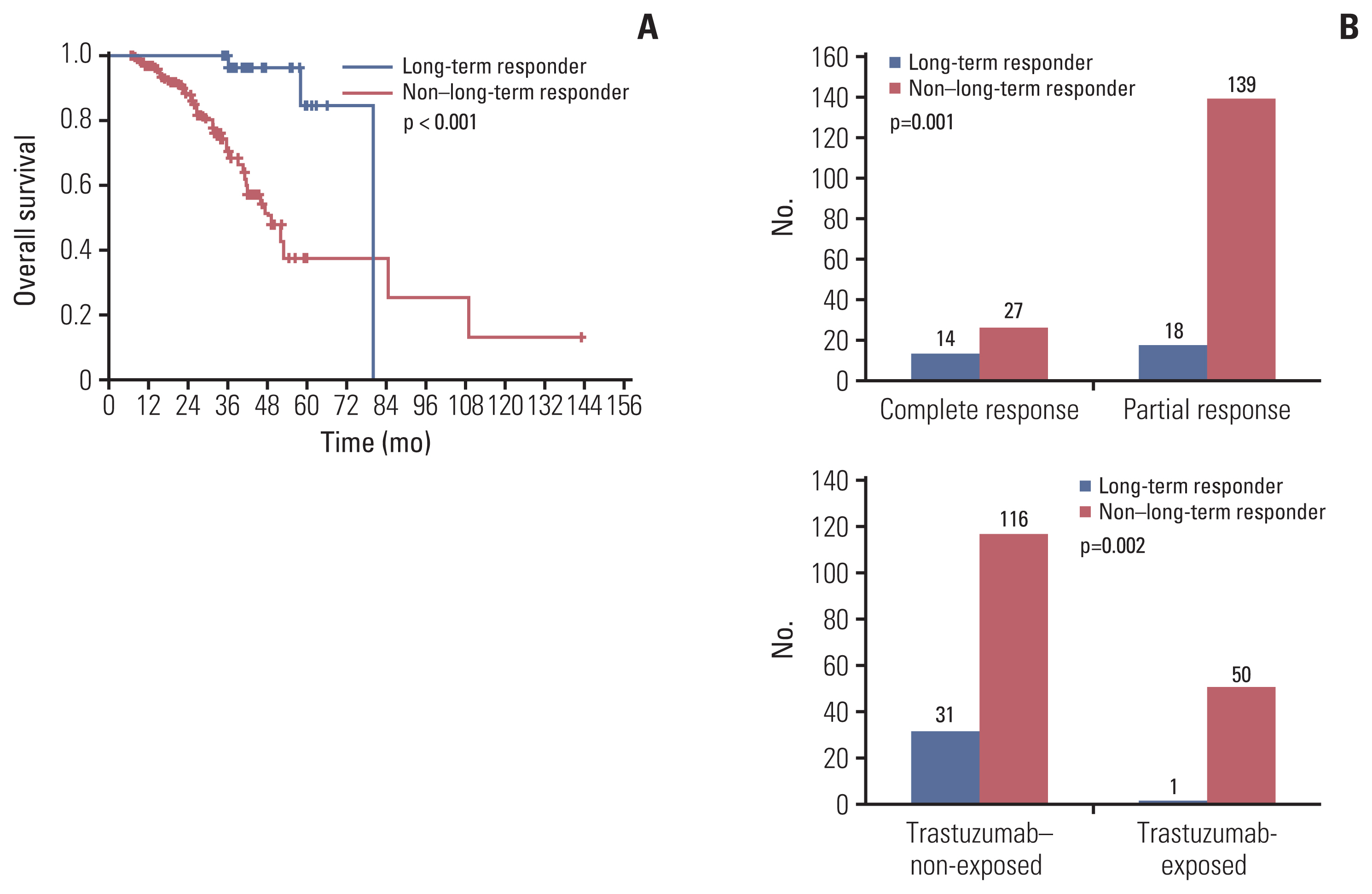
After a median follow-up duration of 28.7 months, median overall survival and progression-free survival were 58.3 months (95% confidence interval [CI], 36.6 to 80.0) and 19.1 months (95% CI, 16.2 to 21.9), respectively. Better survival outcomes were observed in patient who received docetaxel for more than six cycles. Patients exposed to anti-HER2 directed therapies in a perioperative setting had poor survival outcomes. The overall response rate was 86.8% with a complete response (CR) rate of 17.7%. Among responders, 16.7% of patients sustained THP over 35 months and showed better survivals and higher CR rates. Adverse events were comparable to those reported in previous studies.
Conclusion
In a real-world context, clinical outcomes of Korean HER2-positive MBC patients treated with THP were similar to those of patients in the CLEOPATRA trial. Much longer follow-up results would be warranted.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009; 14:320–68.
Article3. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–82.
Article4. Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med. 2007; 357:39–51.
Article5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.
Article6. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009; 9:463–75.
Article7. Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008; 68:5878–87.8. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2011; 366:109–19.
Article9. Swain SM, Ewer MS, Cortes J, Amadori D, Miles D, Knott A, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013; 18:257–64.
Article10. Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013; 14:461–71.
Article11. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015; 372:724–34.
Article12. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21:519–30.13. Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016; 62:132–7.
Article14. US Departement of Health and Human Servies. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [Internet]. Washington, DC: U.S. Departement of Health and Human Servies;2017. [cited 2019 Feb 26]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf .15. Takahashi M, Ohtani S, Nagai SE, Takashima S, Yamaguchi M, Tsuneizumi M, et al. The efficacy and safety of pertuzumab plus trastuzumab and docetaxel as a first-line therapy in Japanese patients with inoperable or recurrent HER2-positive breast cancer: the COMACHI study. Breast Cancer Res Treat. 2021; 185:125–34.
Article16. Gamucci T, Pizzuti L, Natoli C, Mentuccia L, Sperduti I, Barba M, et al. A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol Ther. 2019; 20:192–200.
Article17. Ramagopalan SV, Pisoni R, Rathore LS, Ray J, Sammon C. Association of pertuzumab, trastuzumab, and docetaxel combination therapy with overall survival in patients with metastatic breast cancer. JAMA Netw Open. 2021; 4:e2027764.
Article18. Wong Y, Raghavendra AS, Hatzis C, Irizarry JP, Vega T, Horowitz N, et al. Long-term survival of de novo stage IV human epidermal growth receptor 2 (HER2) positive breast cancers treated with HER2-targeted therapy. Oncologist. 2019; 24:313–8.
Article19. Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017; 18:732–42.
Article20. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367:1783–91.
Article21. Uncu D, Bayoglu IV, Arslan UY, Kucukoner M, Artac M, Koca D, et al. Efficacy of trastuzumab-based therapy after disease progression on lapatinib based therapy in heavily pretreated HER2-positive metastatic breast cancer patients. J Clin Oncol. 2014; 32(15 Suppl):e11586.
Article22. Lang I, Bell R, Feng FY, Lopez RI, Jassem J, Semiglazov V, et al. Trastuzumab retreatment after relapse on adjuvant trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer: final results of the Retreatment after HErceptin Adjuvant trial. Clin Oncol (R Coll Radiol). 2014; 26:81–9.
Article23. Xu B, Hu XC, Zheng H, Wang X, Zhang Q, Cui S, et al. Outcomes of re-treatment with first-line trastuzumab plus taxane in patients (pts) with metastatic breast cancer (mBC) who relapsed after (neo)adjuvant trastuzumab: a prospective multicenter study. J Clin Oncol. 2016; 34(15 Suppl):e12068.
Article24. Rier HN, Levin MD, van Rosmalen J, Bos M, Drooger JC, de Jong P, et al. First-line palliative HER2-targeted therapy in HER2-positive metastatic breast cancer is less effective after previous adjuvant trastuzumab-based therapy. Oncologist. 2017; 22:901–9.
Article25. von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019; 380:617–28.
Article26. Tripathy D, Brufsky A, Cobleigh M, Jahanzeb M, Kaufman PA, Mason G, et al. De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs Registry. Oncologist. 2020; 25:e214–22.
Article27. Gradishar WJ. Taxanes for the treatment of metastatic breast cancer. Breast Cancer (Auckl). 2012; 6:159–71.
Article28. Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005; 352:2302–13.
Article29. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012; 118:2250–60.
Article30. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020; 382:610–21.
Article31. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020; 382:597–609.
Article32. Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Musolino A, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021; 7:573–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trastuzumab Biosimilar (HLX02), Pertuzumab Plus Chemotherapy in Patients with HER2-Positive Metastatic Breast Cancer after Progression of Trastuzumab: A Prospective, Phase II Study
- Real World Evidence of Neoadjuvant Docetaxel/Carboplatin/Trastuzumab/Pertuzumab (TCHP) in Patients with HER2-Positive Early or Locally Advanced Breast Cancer: A Single-Institutional Clinical Experience
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination