Cancer Res Treat.
2016 Apr;48(2):843-847. 10.4143/crt.2014.234.
Intrathecal Trastuzumab Treatment in Patients with Breast Cancer and Leptomeningeal Carcinomatosis
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Soonchunhyang University Hospital Seoul, Seoul, Korea.
- 2Division of Hematology-Oncology, Department of Internal Medicine, Soonchunhyang University Hospital Cheonan, Cheonan, Korea. sclee@schmc.ac.kr
- KMID: 2454364
- DOI: http://doi.org/10.4143/crt.2014.234
Abstract
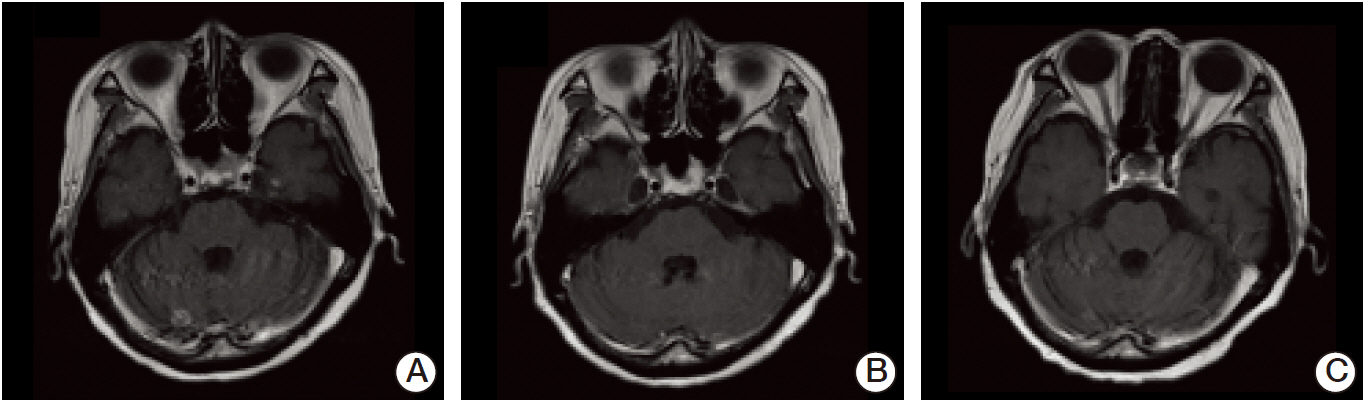
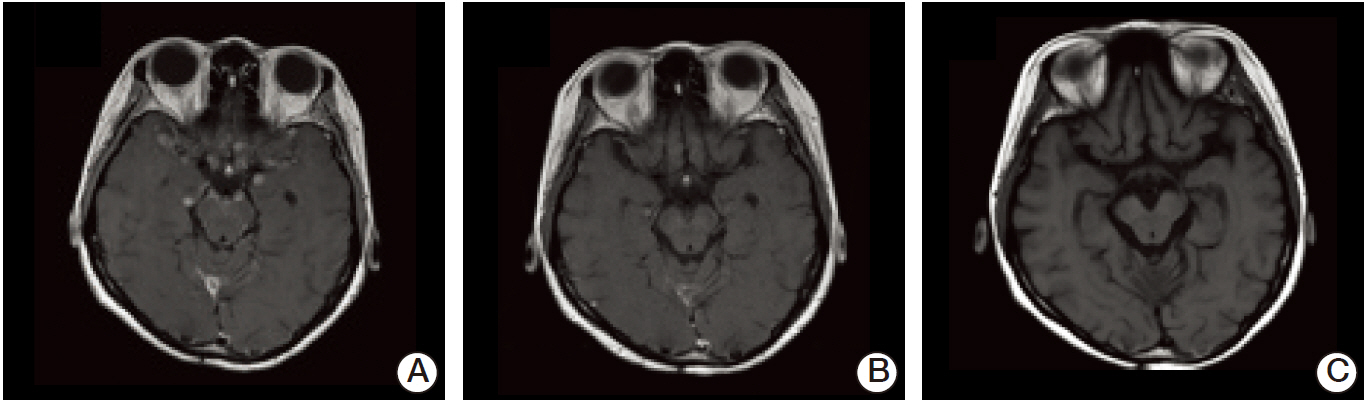
- Leptomeningeal carcinomatosis is a fatal manifestation of metastatic breast cancer. Investigation of intrathecal (IT) trastuzumab for leptomeningeal carcinomatosis is currently underway; however, there has been no consensus. We report on two cases of human epidermal growth factor receptor 2 positive (HER2+) breast cancer following IT trastuzumab for leptomeningeal carcinomatosis. The first patient was treated with weekly IT 15 mg methotrexate plus IT 50 mg trastuzumab for 7 months, followed by IT trastuzumab (50 mg > 25 mg) for 18 months. The other patient received IT trastuzumab with systemic chemotherapy (trastuzumab and/or paclitaxel) for 13 months. Good control of leptomeningeal disease was achieved with IT trastuzumab in both patients, with survival durations of 20 and 29 months, respectively. We suggest that IT trastuzumab is a promising treatment for patients with HER2+ breast cancer and leptomeningeal carcinomatosis.
MeSH Terms
Figure
Reference
-
References
1. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999; 25:103–19.
Article2. Jayson GC, Howell A. Carcinomatous meningitis in solid tumours. Ann Oncol. 1996; 7:773–86.
Article3. Pienkowski T, Zielinski CC. Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2010; 21:917–24.4. Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013; 139:13–22.
Article5. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010; 74:1449–54.
Article6. Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006; 11 Suppl 1:4–12.
Article7. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000; 18:2349–51.
Article8. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007; 18:23–8.
Article9. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013; 4(Suppl 4):S265–88.
Article10. Mir O, Ropert S, Alexandre J, Lemare F, Goldwasser F. High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol. 2008; 19:1978–80.
Article11. Hofer S, Mengele K, Stemmler HJ, Schmitt M, Pestalozzi B. Intrathecal trastuzumab: dose matters. Acta Oncol. 2012; 51:955–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leptomeningeal Carcinomatosis in Solid Tumors; Clinical Manifestation and Treatment
- Breast Cancer with Leptomeningeal Metastasis
- A Case of Leptomeningeal Carcinomatosis Presenting as a Neurological Complication of Stomach Cancer
- Trastuzumab Exposure during Pregnancy with Invasive Breast Cancer
- Brain Metastasis and Leptomeningeal Carcinomatosis in Breast Cancer