J Periodontal Implant Sci.
2020 Feb;50(1):14-27. 10.5051/jpis.2020.50.1.14.
Distinctive bone regeneration of calvarial defects using biphasic calcium phosphate supplemented ultraviolet-crosslinked collagen membrane
- Affiliations
-
- 1Department of Periodontology, Research Institute of Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- KMID: 2470827
- DOI: http://doi.org/10.5051/jpis.2020.50.1.14
Abstract
- PURPOSE
To overcome several drawbacks of chemically-crosslinked collagen membranes, modification processes such as ultraviolet (UV) crosslinking and the addition of biphasic calcium phosphate (BCP) to collagen membranes have been introduced. This study evaluated the efficacy and biocompatibility of BCP-supplemented UV-crosslinked collagen membrane for guided bone regeneration (GBR) in a rabbit calvarial model.
METHODS
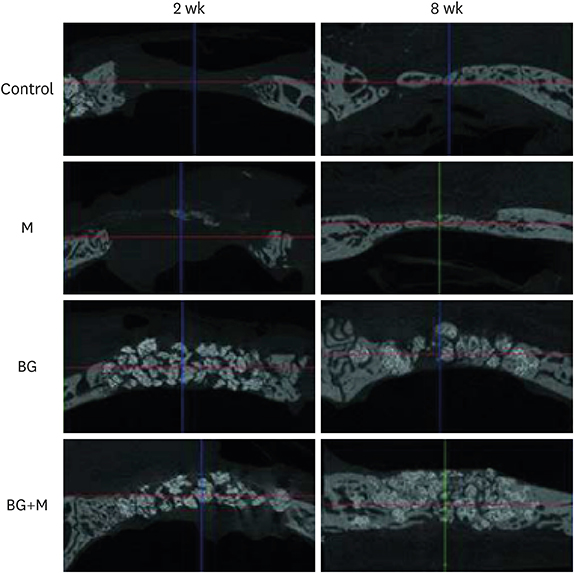
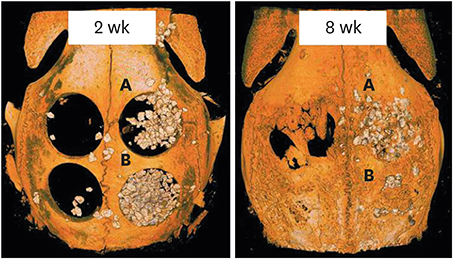
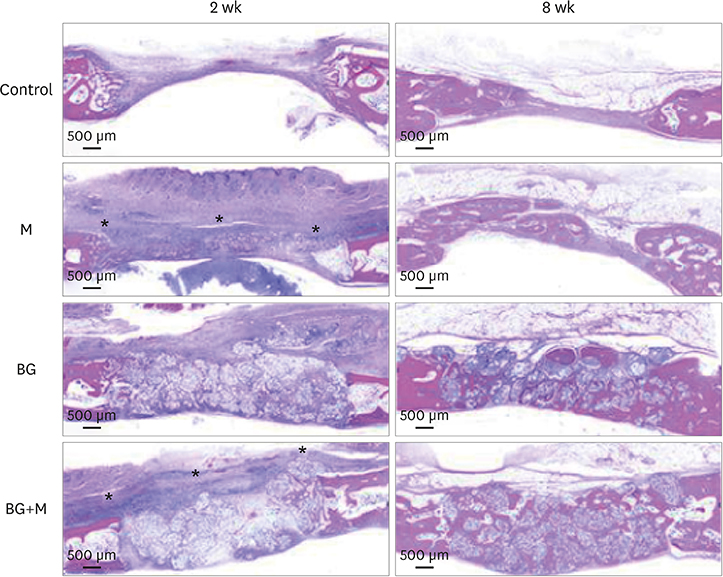
Four circular bone defects (diameter, 8 mm) were created in the calvarium of 10 rabbits. Each defect was randomly allocated to one of the following groups: 1) the sham control group (spontaneous healing); 2) the M group (defect coverage with a BCP-supplemented UV-crosslinked collagen membrane and no graft material); 3) the BG (defects filled with BCP particles without membrane coverage); and 4) the BG+M group (defects filled with BCP particles and covered with a BCP-supplemented UV-crosslinked collagen membrane in a conventional GBR procedure). At 2 and 8 weeks, rabbits were sacrificed, and experimental defects were investigated histologically and by micro-computed tomography (micro-CT).
RESULTS
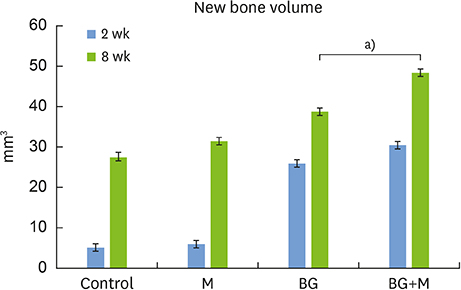
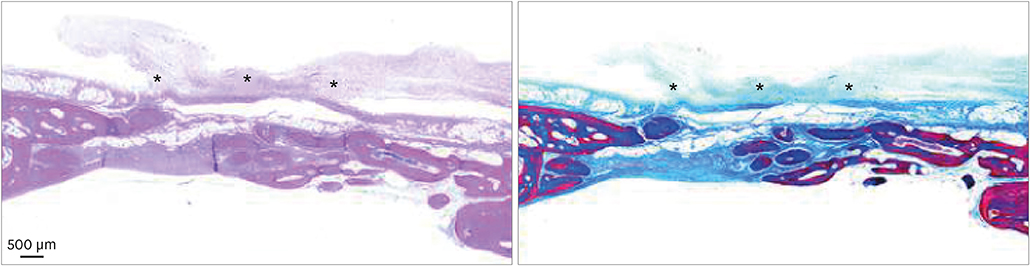
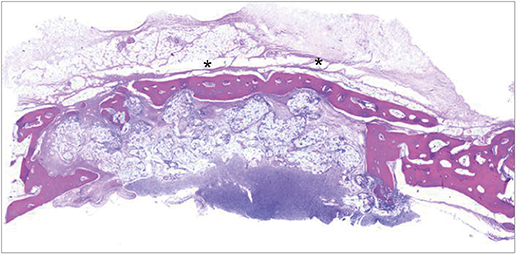
In both micro-CT and histometric analyses, the BG and BG+M groups at both 2 and 8 weeks showed significantly higher new bone formation than the control group. On micro-CT, the new bone volume of the BG+M group (48.39±5.47 mm3) was larger than that of the BG group (38.71±2.24 mm3, P=0.032) at 8 weeks. Histologically, greater new bone area was observed in the BG+M group than in the BG or M groups. BCP-supplemented UV-crosslinked collagen membrane did not cause an abnormal cellular reaction and was stable until 8 weeks.
CONCLUSIONS
Enhanced new bone formation in GBR can be achieved by simultaneously using bone graft material and a BCP-supplemented UV-crosslinked collagen membrane, which showed high biocompatibility and resistance to degradation, making it a biocompatible alternative to chemically-crosslinked collagen membranes.
Keyword
MeSH Terms
Figure
Reference
-
1. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81:672–676.
Article2. Hämmerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003; 33:36–53.
Article3. Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001; 72:215–229.
Article4. Retzepi M, Donos N. Guided bone regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010; 21:567–576.
Article5. Olde Damink LH, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995; 6:460–472.
Article6. An YZ, Heo YK, Lee JS, Jung UW, Choi SH. Dehydrothermally cross-linked collagen membrane with a bone graft improves bone regeneration in a rat calvarial defect model. Materials (Basel). 2017; 10:E927.
Article7. Park JY, Jung IH, Kim YK, Lim HC, Lee JS, Jung UW, et al. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater Res. 2015; 19:15.
Article8. Bornstein MM, Heynen G, Bosshardt DD, Buser D. Effect of two bioabsorbable barrier membranes on bone regeneration of standardized defects in calvarial bone: a comparative histomorphometric study in pigs. J Periodontol. 2009; 80:1289–1299.
Article9. Angele P, Abke J, Kujat R, Faltermeier H, Schumann D, Nerlich M, et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials. 2004; 25:2831–2841.
Article10. Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987; 21:741–771.
Article11. Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005; 16:369–378.
Article12. Wiebe D, Megerman J, L'Italien GJ, Abbott WM. Glutaraldehyde release from vascular prostheses of biologic origin. Surgery. 1988; 104:26–33.13. Davidenko N, Bax DV, Schuster CF, Farndale RW, Hamaia SW, Best SM, et al. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J Mater Sci Mater Med. 2016; 27:14.
Article14. LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003; 14:201–209.15. Song JH, Kim HE, Kim HW. Collagen-apatite nanocomposite membranes for guided bone regeneration. J Biomed Mater Res B Appl Biomater. 2007; 83:248–257.
Article16. Kitayama S, Wong LO, Ma L, Hao J, Kasugai S, Lang NP, et al. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin Oral Implants Res. 2016; 27:e206–14.
Article17. Pae HC, Kang JH, Cha JK, Lee JS, Paik JW, Jung UW, et al. 3D-printed polycaprolactone scaffold mixed with β-tricalcium phosphate as a bone regenerative material in rabbit calvarial defects. J Biomed Mater Res B Appl Biomater. 2019; 107:1254–1263.
Article18. Fugazzotto PA. GBR using bovine bone matrix and resorbable and nonresorbable membranes. Part 1: histologic results. Int J Periodontics Restorative Dent. 2003; 23:361–369.19. Zitzmann NU, Naef R, Schärer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants. 1997; 12:844–852.20. Jung RE, Fenner N, Hämmerle CH, Zitzmann NU. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin Oral Implants Res. 2013; 24:1065–1073.
Article21. Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent Mater. 2012; 28:703–721.
Article22. Schwarz F, Rothamel D, Herten M, Wüstefeld M, Sager M, Ferrari D, et al. Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: an experimental study in dogs. Clin Oral Implants Res. 2008; 19:402–415.
Article23. Nooh N, Ramalingam S, Al-Kindi M, Al-Rasheed A, Al-Hamdan KS, Al-Hezaimi K. Real-time assessment of guided bone regeneration in standardized calvarial defects in rats using Bio-Oss with and without collagen membrane: an in vivo microcomputed tomographic and histologic experiment. Int J Periodontics Restorative Dent. 2016; 36 Suppl:s139–s149.24. Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002; 23:1205–1212.
Article25. Hao J, Acharya A, Chen K, Chou J, Kasugai S, Lang NP. Novel bioresorbable strontium hydroxyapatite membrane for guided bone regeneration. Clin Oral Implants Res. 2015; 26:1–7.
Article26. Zubery Y, Goldlust A, Alves A, Nir E. Ossification of a novel cross-linked porcine collagen barrier in guided bone regeneration in dogs. J Periodontol. 2007; 78:112–121.
Article27. Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, et al. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials. 2005; 26:6158–6166.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diverse patterns of bone regeneration in rabbit calvarial defects depending on the type of collagen membrane
- The Effects of Calcium-Phosphate Coated Xenogenic Bone and Type I Collagen for Bone Regeneration on the Calvarial Defects in Rabbits
- The biological effect of cyanoacrylate-combined calcium phosphate in rabbit calvarial defects
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- Decellularized Non-cross-linked Collagen Membranes for Guided Bone Regeneration in Rabbit Calvarial Defects