J Periodontal Implant Sci.
2011 Jun;41(3):123-130. 10.5051/jpis.2011.41.3.123.
The biological effect of cyanoacrylate-combined calcium phosphate in rabbit calvarial defects
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2YESBIO Co., Seoul, Korea.
- KMID: 1783602
- DOI: http://doi.org/10.5051/jpis.2011.41.3.123
Abstract
- PURPOSE
The purpose of this study was to determine the biological effects of cyanoacrylate-combined calcium phosphate (CCP), in particular its potential to act as a physical barrier - functioning like a membrane - in rabbit calvarial defects.
METHODS
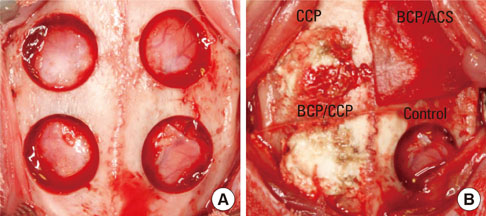
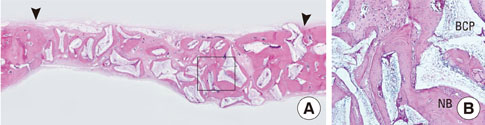
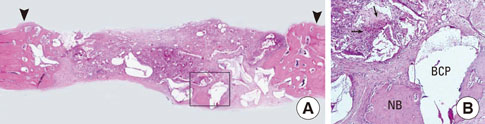
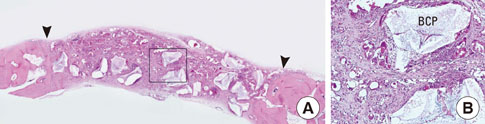
In each animal, four circular calvarial defects with a diameter of 8 mm were prepared and then filled with either nothing (control group) or one of three different experimental materials. In the experimental conditions, they were filled with CCP alone (CCP group), filled with biphasic calcium phosphate (BCP) and then covered with an absorbable collagen sponge (ACS; BCP/ACS group), or filled with BCP and then covered by CCP (BCP/CCP group).
RESULTS
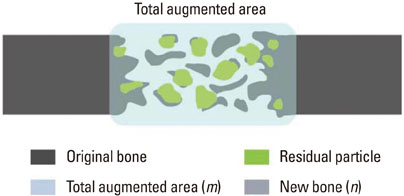
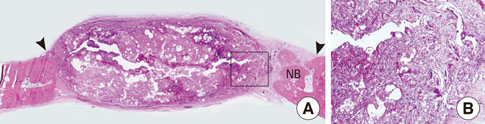
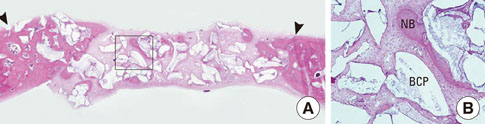
After 4 and 8 weeks of healing, new bone formation appeared to be lower in the CCP group than in the control group, but the difference was not statistically significant. In both the CCP and BCP/CCP groups, inflammatory cells could be seen after 4 and 8 weeks of healing.
CONCLUSIONS
Within the limits of this study, CCP exhibited limited osteoconductivity in rabbit calvarial defects and was histologically associated with the presence of inflammatory cells. However, CCP demonstrated its ability to stabilize graft particles and its potential as an effective defect filler in bone augmentation, if the biocompatibility and osteoconductivity of CCP were improved.
MeSH Terms
Figure
Cited by 1 articles
-
Biomimetic characteristics of mussel adhesive protein-loaded collagen membrane in guided bone regeneration of rabbit calvarial defects
Woong-Kyu Song, Joo-Hyun Kang, Jae-Kook Cha, Jung-Seok Lee, Jeong-Won Paik, Ui-Won Jung, Byung-Hoon Kim, Seong-Ho Choi
J Periodontal Implant Sci. 2018;48(5):305-316. doi: 10.5051/jpis.2018.48.5.305.
Reference
-
1. Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials - biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 2008. 35:8 Suppl. 106–116.
Article2. Nkenke E, Stelzle F. Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin Oral Implants Res. 2009. 20:Suppl 4. 124–133.
Article3. Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009. 20:Suppl 4. 113–123.
Article4. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004. 187:238–248.
Article5. Brown JK, Campbell BT, Drongowski RA, Alderman AK, Geiger JD, Teitelbaum DH, et al. A prospective, randomized comparison of skin adhesive and subcuticular suture for closure of pediatric hernia incisions: cost and cosmetic considerations. J Pediatr Surg. 2009. 44:1418–1422.
Article6. Al-Belasy FA, Amer MZ. Hemostatic effect of n-butyl-2-cyanoacrylate (histoacryl) glue in warfarin-treated patients undergoing oral surgery. J Oral Maxillofac Surg. 2003. 61:1405–1409.
Article7. Quinn J, Maw J, Ramotar K, Wenckebach G, Wells G. Octylcyanoacrylate tissue adhesive versus suture wound repair in a contaminated wound model. Surgery. 1997. 122:69–72.
Article8. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005. 23:193–198.
Article9. Singer AJ, Nable M, Cameau P, Singer DD, McClain SA. Evaluation of a new liquid occlusive dressing for excisional wounds. Wound Repair Regen. 2003. 11:181–187.
Article10. Singer A, Thode H Jr, McClain S. The effects of octylcyanoacrylate on scarring after burns. Acad Emerg Med. 2001. 8:107–111.
Article11. Kutcher M. Evaluating the efficacy of 2-octyl cyanoacrylate bioadhesive for treatment of oral ulcerations. Compend Contin Educ Dent Suppl. 2001. (32):12–16.12. Bhaskar SN, Cutright DE, Beasley JD, Ward JP. Oral spray of isobutyl cyanoacrylate and its systemic effect. Oral Surg Oral Med Oral Pathol. 1970. 29:313–319.
Article13. Lee SB, Park KJ, Lee DY, Park JJ, Hwang JS, Lee YK, et al. A histological evaluation of novel cyanoacrylate-based β-TCP composite in rat calvarial defects. Key Eng Mater. 2006. 309-311:1133–1136.
Article14. Park KJ, Park JH, Lee SB, Lee DY, Kim KN, Kim KM, et al. Bioactive cyanoacrylate-based filling material for bone defects in dental applications. Key Eng Mater. 2005. 284-6:933–936.
Article15. Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg. 2007. 77:209–213.
Article16. Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006. 17:237–243.
Article17. Bowers GM, Vargo JW, Levy B, Emerson JR, Bergquist JJ. Histologic observations following the placement of tricalcium phosphate implants in human intrabony defects. J Periodontol. 1986. 57:286–287.
Article18. Im JS, Jung UW, Chang YY, Yeon JY, Um YJ, Kim CS, et al. The application of cyanoacrylate-based filling material for surgically created 1-wall intrabony defects in dogs. Biomater Res. 2009. 13:128–132.19. Dragu A, Unglaub F, Schwarz S, Beier JP, Kneser U, Bach AD, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009. 129:167–169.
Article20. Rengstorff DS, Binmoeller KF. A pilot study of 2-octyl cyanoacrylate injection for treatment of gastric fundal varices in humans. Gastrointest Endosc. 2004. 59:553–558.
Article21. Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, et al. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci. 2008. 53:2258–2267.
Article22. Losanoff JE, Richman BW, Jones JW. Cyanoacrylate adhesive in management of severe presacral bleeding. Dis Colon Rectum. 2002. 45:1118–1119.
Article23. Kim YK, Yun PY, Lim SC, Kim SG, Lee HJ, Ong JL. Clinical evaluations of OSTEON as a new alloplastic material in sinus bone grafting and its effect on bone healing. J Biomed Mater Res B Appl Biomater. 2008. 86:270–277.
Article24. Um YJ, Hong JY, Kim ST, Lee YH, Park SH, Park SH, et al. Bone formation of newly developed biphasic calcium phosphate in rabbit calvarial defect model: a pilot study. J Korean Acad Periodontol. 2008. 38:163–170.
Article25. Fleckenstein KB, Cuenin MF, Peacock ME, Billman MA, Swiec GD, Buxton TB, et al. Effect of a hydroxyapatite tricalcium phosphate alloplast on osseous repair in the rat calvarium. J Periodontol. 2006. 77:39–45.
Article26. Chang YY, Jung UW, Im JS, Yon JY, Um YJ, Kim CS, et al. The effects of hydroxyapatite coated with beta -tricalcium phosphate in one-wall intrabony defects in dogs. Biomater Res. 2010. 14:111–117.27. Moreira-Gonzalez A, Lobocki C, Barakat K, Andrus L, Bradford M, Gilsdorf M, et al. Evaluation of 45S5 bioactive glass combined as a bone substitute in the reconstruction of critical size calvarial defects in rabbits. J Craniofac Surg. 2005. 16:63–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- The Effects of Calcium-Phosphate Coated Xenogenic Bone and Type I Collagen for Bone Regeneration on the Calvarial Defects in Rabbits
- The effects of novel biodegradable amorphous Calcium Phosphate on bone regeneration in rat calvarial defects
- Effectiveness of biphasic calcium phosphate block bone substitutes processed using a modified extrusion method in rabbit calvarial defects
- Histomorphometric evaluation of bone healing with fully interconnected microporous biphasic calcium phosphate ceramics in rabbit calvarial defects