Korean J Ophthalmol.
2020 Feb;34(1):1-10. 10.3341/kjo.2019.0046.
Effect of Diquafosol on Hyperosmotic Stress-induced Tumor Necrosis Factor-α and Interleukin-6 Expression in Human Corneal Epithelial Cells
- Affiliations
-
- 1Myungmoon Bio, Hwaseong, Korea.
- 2Department of Physiology, College of Korean Medicine Dongguk University, Gyeongju, Korea.
- 3Binaree, Daegu, Korea.
- 4Central Ophthalmic Clinic, Daegu, Korea. eyepark9@naver.com
- 5Division of Biomedicinal & Cosmetics, College of Sciences & Technology, Mokwon University, Daejeon, Korea.
- 6Developmental Biology Laboratory, Department of Biology, College of Natural Sciences, Kyungpook National University, Daegu, Korea. jcjung@knu.ac.kr
- KMID: 2469281
- DOI: http://doi.org/10.3341/kjo.2019.0046
Abstract
- PURPOSE
Diquafosol is a pharmaceutical drug used for dry eye treatment with a novel mechanism of action. It is a purinergic P2Y2 receptor agonist that promotes the secretion of tears and healing of corneal epithelial wounds. However, its inhibitory effect on hyperosmotic stress-induced inflammation in human corneal epithelial cells (HCECs) remains unclear.
METHODS
A hyperosmotic stress model was established by transferring HCECs from isosmotic (312 mOsm/kg to hyperosmotic medium (500 mOsm/kg). HCECs were incubated with 500 mOsm/kg hyperosmotic medium for 30 minutes, and then treated with diquafosol (0.6-6 mg/mL) for 4 or 24 hours. Cells were then harvested and analyzed by western blot, immunocytochemistry, and real-time polymerase chain reaction to evaluate the expression of interleukin-6, tumor necrosis factor-alpha, and the phosphorylation status of nuclear factor-kappa B.
RESULTS
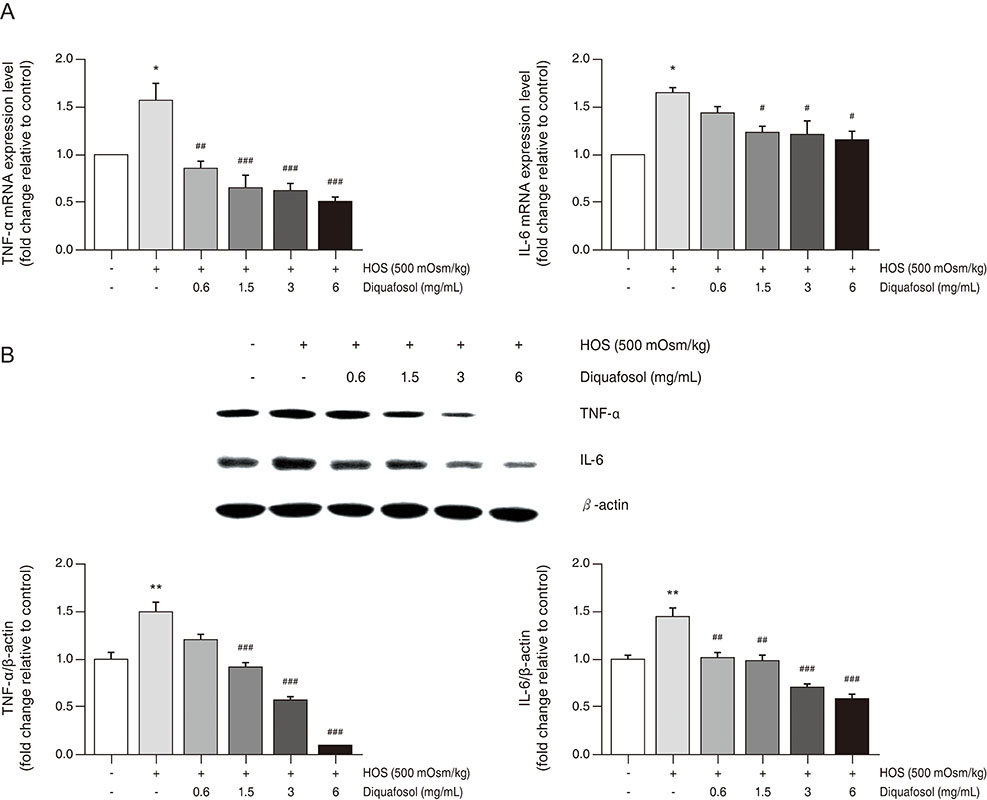
Diquafosol significantly decreased the mRNA and protein expression of hyperosmotic stress-induced tumor necrosis factor-alpha and interleukin-6. These results were supported by immunofluorescence staining and quantitative real-time polymerase chain reaction analysis. Furthermore, diquafosol inhibits nuclear factor-kappa B activation by suppressing the phosphorylation and degradation of the inhibitor of кB.
CONCLUSIONS
This study shows that diquafosol inhibits nuclear factor-kappa B signaling and inflammatory factors induced by hyperosmotic stress in HCECs. This suggests that using diquafosol for the improvement of dry eye syndrome could be effective in the treatment of inflammation-related corneal and conjunctival diseases.
Keyword
MeSH Terms
-
Blotting, Western
Conjunctival Diseases
Dry Eye Syndromes
Epithelial Cells*
Fluorescent Antibody Technique
Humans*
Immunohistochemistry
Inflammation
Interleukin-6*
Necrosis*
Phosphorylation
Real-Time Polymerase Chain Reaction
RNA, Messenger
Tears
Tumor Necrosis Factor-alpha
Wounds and Injuries
Interleukin-6
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Gulati S, Jain S. Ocular pharmacology of tear film, dry eye, and allergic conjunctivitis. Handb Exp Pharmacol. 2017; 242:97–118.
Article2. McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998; 105:1114–1119.3. Kuang TM, Tsai SY, Hsu WM, et al. Correctable visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2007; 48:1032–1037.
Article4. Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007; 143:409–415.5. Versura P, Profazio V, Schiavi C, Campos EC. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol Vis Sci. 2011; 52:5488–5496.
Article6. Bellotti M, Bast W, Berra A, Bonetto FJ. Effects of osmolarity on human epithelial conjunctival cells using an electrical technique. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1875–1882.
Article7. Julio G, Lluch S, Pujol P, Merindano MD. Effects of tear hyperosmolarity on conjunctival cells in mild to moderate dry eye. Ophthalmic Physiol Opt. 2012; 32:317–323.
Article8. Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45:4302–4311.
Article9. Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006; 82:588–596.10. Cavet ME, Harrington KL, Ward KW, Zhang JZ. Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells. Mol Vis. 2010; 16:1791–1800.11. Al-Ayyoubi S, Gali-Muhtasib H. Differential apoptosis by gallotannin in human colon cancer cells with distinct p53 status. Mol Carcinog. 2007; 46:176–186.
Article12. Neuhofer W. Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genomics. 2010; 11:584–590.
Article13. Schwartz L, Guais A, Pooya M, Abolhassani M. Is inflammation a consequence of extracellular hyperosmolarity? J Inflamm (Lond). 2009; 6:21.
Article14. Nichols KK, Yerxa B, Kellerman DJ. Diquafosol tetrasodium: a novel dry eye therapy. Expert Opin Investig Drugs. 2004; 13:47–54.
Article15. Gum SI, Kim YH, Jung JC, et al. Cyclosporine A inhibits TGF-β2-induced myofibroblasts of primary cultured human pterygium fibroblasts. Biochem Biophys Res Commun. 2017; 482:1148–1153.
Article16. Kanellopoulos AJ, Asimellis G. In pursuit of objective dry eye screening clinical techniques. Eye Vis (Lond). 2016; 3:1.
Article17. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the d iagnosis and management of d ry eye d isease. Am J Ophthalmol. 2011; 151:792–798.18. Corrales RM, Villarreal A, Farley W, et al. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007; 26:579–584.
Article19. Gilbard JP, Carter JB, Sang DN, et al. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology. 1984; 91:1205–1212.
Article20. Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005; 31:186–193.
Article21. Pan Z, Wang Z, Yang H, et al. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011; 52:485–493.
Article22. Lam H, Bleiden L, de Paiva CS, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009; 147:198–205.
Article23. Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010; 16:862–873.24. Koh S. Clinical utility of 3% diquafosol ophthalmic solution in the treatment of dry eyes. Clin Ophthalmol. 2015; 9:865–872.
Article25. Park JH, Moon SH, Kang DH, et al. Diquafosol sodium inhibits apoptosis and inf lammation of corneal epithelial cells via activation of Erk1/2 and RSK: in vitro and in vivo dry eye model. Invest Ophthalmol Vis Sci. 2018; 59:5108–5115.26. Lan W, Petznick A, Heryati S, et al. Nuclear Factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012; 10:137–148.
Article27. Shi H, Berger EA. Characterization of site-specific phosphorylation of NF-κB p65 in retinal cells in response to high glucose and cytokine polarization. Mediators Inflamm. 2018; 2018:3020675.
Article28. Guzman M, Keitelman I, Sabbione F, et al. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin Exp Immunol. 2016; 184:248–256.
Article29. Fujihara T, Murakami T, Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.30. Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009; 28:1023–1027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Tumor Necrosis Factor Alpha, Interleukin 8 and Dexamethasone in the FAK Expression by Human Nucleus Pulposus Cells
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- The Effect of the Corneal Epithelium on the Keratocyte Apoptosis
- Effects of Platelet-rich Plasma on Ocular Surface in Patients with Dry Eye Syndrome: Clinico-experimental Analysis
- CXC and CC Chemokine Expression by Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A