J Bacteriol Virol.
2019 Dec;49(4):221-229. 10.4167/jbv.2019.49.4.221.
p90RSK Activation Promotes Epithelial-Mesenchymal Transition in Cisplatin-Treated Triple-Negative Breast Cancer Cells
- Affiliations
-
- 1College of Pharmacy and Institute of Drug Research and Development, Chungnam National University, Daejeon, South Korea. kheo@cnu.ac.kr
- KMID: 2468028
- DOI: http://doi.org/10.4167/jbv.2019.49.4.221
Abstract
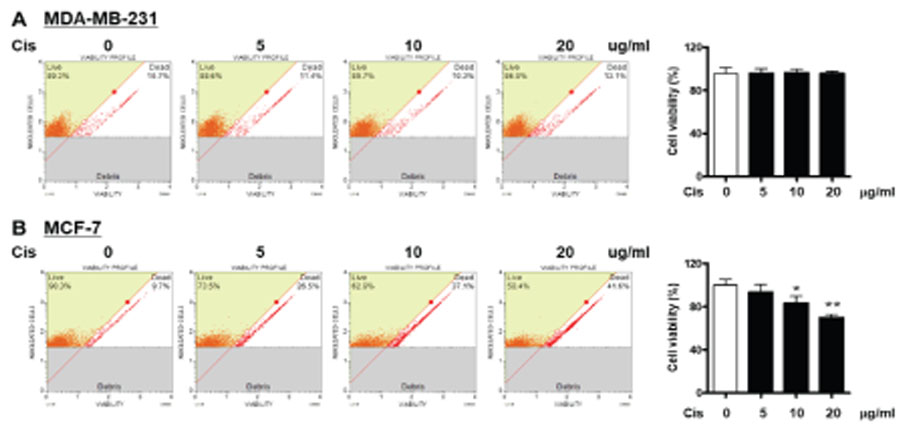
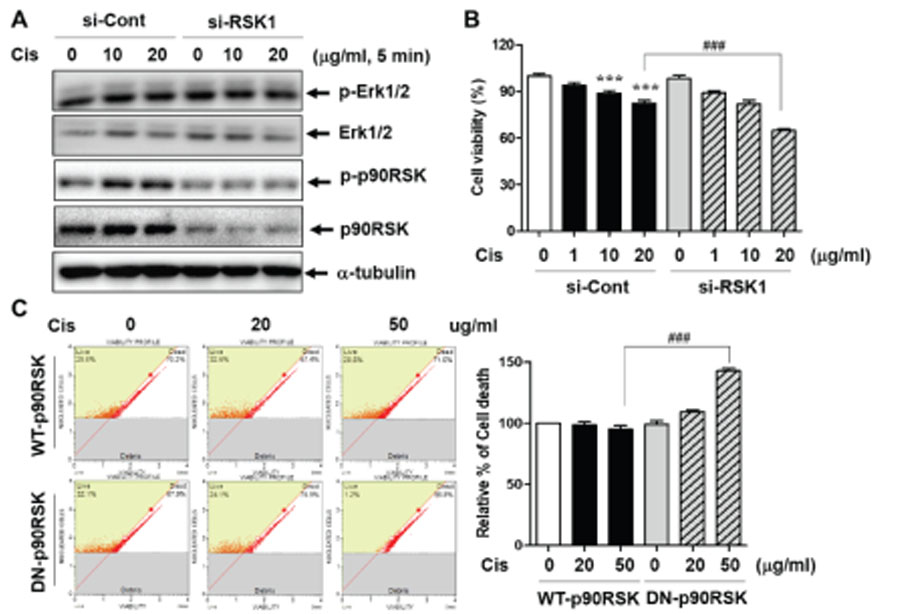
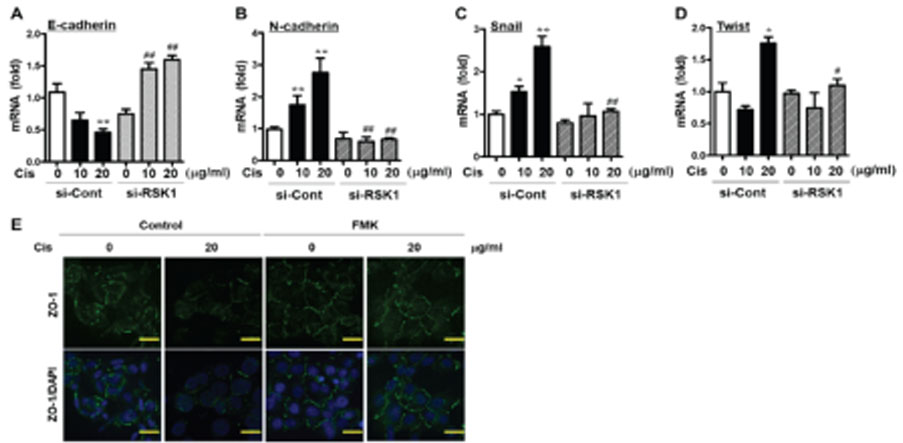
- p90 ribosomal S6 kinase (p90RSK), one of the downstream effectors in ERK1/2 pathways, shows high expression in human breast cancer tissues. However, its role in breast cancer development and drug resistance is not fully understood. Here, we demonstrate that Cis-DDP treatment failed to increase cytotoxicity in MDA-MB-231 cells compared to MCF-7 cells and p90RSK activation was involved in Cis-DDP-resistance to MDA-MB-231 cells. In the study, we found that inhibition of p90RSK expression or activation using a small interfering RNA (siRNA) or dominant-negative kinase mutant (DN-p90RSK) plasmid overexpression increased Cis-DDP-induced cytotoxicity of MDA-MB-231 cells, respectively. Mechanistically, we found that Cis-DDP resistance was associated with up-regulation of epithelial growth factor (EGF) expression and EGF treatment induced cancer survival signaling pathway including activation of ERK1/2, p90RSK, and Akt. We also examined the expression of epithelial-mesenchymal transition (EMT)-associated proteins using a reverse transition-quantitative PCR analysis. Cis-DDP treatment induced EMT by increasing the expression levels of N-cadherin, Snail, and Twist, while decreasing the expression levels of E-cadherin. Furthermore, we examined the epithelial marker, Zonula occludens-1 (ZO-1) using immunofluorescence analysis and found that Cis-DDP-inhibited ZO-1 expression was recovered by p90RSK deactivated condition. Therefore, we conclude that Cis-DDP resistance is involved in EMT via regulating the EGF-mediated p90RSK signaling pathway in MDA-MB-231 cells.
Keyword
MeSH Terms
-
Breast Neoplasms
Cadherins
Cisplatin
Drug Resistance
Epidermal Growth Factor
Epithelial-Mesenchymal Transition*
Fluorescent Antibody Technique
Humans
MCF-7 Cells
Phosphotransferases
Plasmids
Polymerase Chain Reaction
Ribosomal Protein S6 Kinases, 90-kDa
RNA, Small Interfering
Snails
Triple Negative Breast Neoplasms*
Up-Regulation
Cadherins
Cisplatin
Epidermal Growth Factor
Phosphotransferases
RNA, Small Interfering
Ribosomal Protein S6 Kinases, 90-kDa
Figure
Reference
-
1. Jung J, Jang K, Ju JM, Lee E, Lee JW, Kim HJ, et al. Novel cancer gene variants and gene fusions of triple-negative breast cancers (TNBCs) reveal their molecular diversity conserved in the patient-derived xenograft (PDX) model. Cancer Lett. 2018; 428:127–138.
Article2. Feng F, Cheng P, Wang C, Wang Y, Wang W. Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol Lett. 2019; 18:5428–5436.
Article3. Melissaridou S, Wiechec E, Magan M, Jain MV, Chung MK, Farnebo L, et al. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019; 19:16.
Article4. Jung KH, Lee EJ, Park JW, Lee JH, Moon SH, Cho YS, et al. EGF receptor stimulation shifts breast cancer cell glucose metabolism toward glycolytic flux through PI3 kinase signaling. PLoS One. 2019; 14:e0221294.
Article5. Pedersen MH, Hood BL, Ehmsen S, Beck HC, Conrads TP, Bak M, et al. CYPOR is a novel and independent prognostic biomarker of recurrence-free survival in triple-negative breast cancer patients. Int J Cancer. 2019; 144:631–640.
Article6. Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL 3rd, Srkalovic G, et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination With Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J Clin Oncol. 2019; 37:222–229.
Article7. Phelip JM, Edeline J, Blanc JF, Barbier E, Michel P, Bourgeois V, et al. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig Liver Dis. 2019; 51:318–320.
Article8. Ludwik KA, Campbell JP, Li M, Li Y, Sandusky ZM, Pasic L, et al. Development of a RSK Inhibitor as a Novel Therapy for Triple-Negative Breast Cancer. Mol Cancer Ther. 2016; 15:2598–2608.
Article9. Lin L, White SA, Hu K. Role of p90RSK in Kidney and Other Diseases. Int J Mol Sci. 2019; 20.
Article10. Huynh DTN, Heo KS. Therapeutic targets for endothelial dysfunction in vascular diseases. Arch Pharm Res. 2019; 42:848–861.
Article11. Chen S, Mackintosh C. Differential regulation of NHE1 phosphorylation and glucose uptake by inhibitors of the ERK pathway and p90RSK in 3T3-L1 adipocytes. Cell Signal. 2009; 21:1984–1993.
Article12. Ikuta M, Kornienko M, Byrne N, Reid JC, Mizuarai S, Kotani H, et al. Crystal structures of the N-terminal kinase domain of human RSK1 bound to three different ligands: Implications for the design of RSK1 specific inhibitors. Protein Sci. 2007; 16:2626–2635.
Article13. Melhuish TA, Kowalczyk I, Manukyan A, Zhang Y, Shah A, Abounader R, et al. Myt1 and Myt1l transcription factors limit proliferation in GBM cells by repressing YAP1 expression. Biochim Biophys Acta Gene Regul Mech. 2018; 1861:983–995.
Article14. Sheng W, Chen C, Dong M, Wang G, Zhou J, Song H, et al. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017; 8:e3147.
Article15. Tas I, Han J, Park SY, Yang Y, Zhou R, Gamage CDB, et al. Physciosporin suppresses the proliferation, motility and tumourigenesis of colorectal cancer cells. Phytomedicine. 2019; 56:10–20.
Article16. Lin S, Zhang C, Liu F, Ma J, Jia F, Han Z, et al. Actinomycin V Inhibits Migration and Invasion via Suppressing Snail/Slug-Mediated Epithelial-Mesenchymal Transition Progression in Human Breast Cancer MDA-MB-231 Cells In Vitro. Mar Drugs. 2019; 17.
Article17. Heo KS, Le NT, Cushman HJ, Giancursio CJ, Chang E, Woo CH, et al. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest. 2015; 125:1299–1310.
Article18. Tang T, Zhu Q, Li X, Zhu G, Deng S, Wang Y, et al. Protease Nexin I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer cells metastasis and stemness. Cell Death Dis. 2019; 10:649.
Article19. Li L, Su N, Zhou T, Zheng D, Wang Z, Chen H, et al. Mixed lineage kinase ZAK promotes epithelial-mesenchymal transition in cancer progression. Cell Death Dis. 2018; 9:143.
Article20. Suen KM, Lin CC, Seiler C, George R, Poncet-Montange G, Biter AB, et al. Phosphorylation of threonine residues on Shc promotes ligand binding and mediates crosstalk between MAPK and Akt pathways in breast cancer cells. Int J Biochem Cell Biol. 2018; 94:89–97.
Article21. Lim W, Yang C, Park S, Bazer FW, Song G. Inhibitory Effects of Quercetin on Progression of Human Choriocarcinoma Cells Are Mediated Through PI3K/AKT and MAPK Signal Transduction Cascades. J Cell Physiol. 2017; 232:1428–1440.
Article22. Singh MV, Kotla S, Le NT, Ae Ko K, Heo KS, Wang Y, et al. Senescent Phenotype Induced by p90RSK-NRF2 Signaling Sensitizes Monocytes and Macrophages to Oxidative Stress in HIV-Positive Individuals. Circulation. 2019; 139:1199–1216.
Article23. Xu M, Wang S, Wang Y, Wu H, Frank JA, Zhang Z, et al. Role of p38gamma MAPK in regulation of EMT and cancer stem cells. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:3605–3617.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Gastric Stem Cells in Gastric Carcinogenesis by Chronic Helicobacter pylori Infection
- Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells
- Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5
- Estradiol-Induced Epithelial to Mesenchymal Transition and Migration Are Inhibited by Blocking c-Src Kinase in Breast Cancer Cell Lines
- Sphingosylphosphorylcholine Induces Thrombospondin-1 Secretion in MCF10A Cells via ERK2